Breaking barriers: Nitric oxide-releasing nanocomplexes for collagen degradation and enhanced αPD-L1 immunotherapy in deep tumor
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
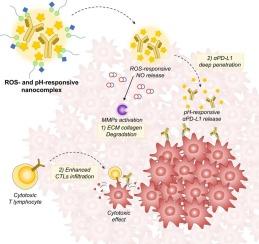
Overcoming the physical barrier of the extracellular matrix (ECM) surrounding tumors is a critical challenge in achieving effective immune checkpoint blockade (ICB). The dense ECM impedes the infiltration of immune checkpoint inhibitors (ICIs) and cytotoxic T lymphocytes (CTLs) into tumor tissues. To address this, we design a nanocomplex incorporating a reactive oxygen species (ROS)-responsive nitric oxide (NO) prodrug around TANNylated αPD-L1. Within the tumor microenvironment (TME), this nanocomplex accumulates and selectively releases NO in response to ROS. The released NO activates matrix metalloproteinases (MMPs) in the ECM, leading to collagen degradation. Following this, the pH-responsive release of αPD-L1 in the deeper tumor regions ensures effective delivery, allowing CTLs to penetrate the tumor more efficiently by bypassing the ECM barrier, thereby enhancing immunotherapy. Overall, this study applies a nanocomplex capable of releasing NO and αPD-L1 in the tumor to a solid tumor model, successfully inhibiting tumor growth by altering the immunosuppressive environment through improved penetration.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


