The Human-Specific miR-6762-5p Is an Activator of RhoA GTPase Enhancing Shigella flexneri Intercellular Spreading
IF 2.6
2区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
MicroRNAs have recently emerged as major players in host –bacterial pathogen interactions, either as part of the host defense mechanism to neutralize infection or as a bacterial arsenal aimed at subverting host cell functions. Here, we identify the newly evolved human microRNA miR-6762-5p as a new player in the host–Shigella interplay. A microarray analysis in infected epithelial cells allowed the detection of this miRNA exclusively during the late phase of infection. Conditional expression of miR-6762-5p combined with a transcriptome analysis indicated a role in cytoskeleton remodeling. Likewise, miR-6762-5p enhanced stress fiber formation through RhoA activation, and in silico analysis identified several regulators of RhoA activity as potential direct transcriptional targets. We further showed that miR-6762-5p expression induces an increase in Shigella intercellular spreading, while miR-6762-5p inhibition reduced bacterial dissemination. We propose a model in which the expression of miR-6762-5p induces cytoskeleton modifications through RhoA activation to achieve a successful dissemination of Shigella in the host.
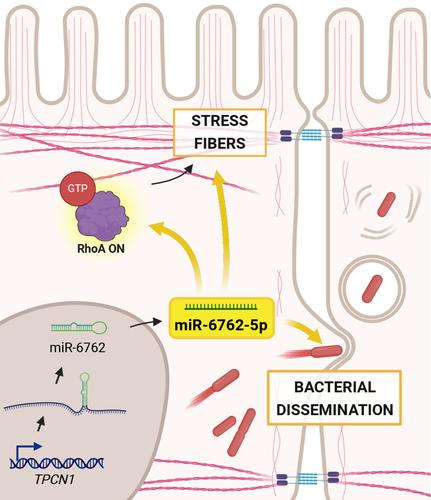
人类特异性miR-6762-5p是RhoA GTPase的激活剂,促进福氏志贺氏菌细胞间传播
最近,microrna在宿主-细菌病原体相互作用中扮演了重要角色,它可以作为宿主防御机制的一部分来中和感染,也可以作为旨在破坏宿主细胞功能的细菌武器库。在这里,我们确定了新进化的人类microRNA miR-6762-5p作为宿主-志贺氏菌相互作用的新参与者。在感染的上皮细胞中进行微阵列分析,可以在感染后期检测到这种miRNA。条件表达miR-6762-5p结合转录组分析表明其在细胞骨架重塑中起作用。同样,miR-6762-5p通过RhoA激活增强应激纤维的形成,并且通过硅分析确定了RhoA活性的几个调节因子作为潜在的直接转录靶点。我们进一步表明,miR-6762-5p表达诱导志贺氏菌细胞间扩散增加,而miR-6762-5p抑制减少细菌传播。我们提出了一个模型,其中miR-6762-5p的表达通过RhoA激活诱导细胞骨架修饰,从而实现志贺氏菌在宿主体内的成功传播。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Microbiology
生物-生化与分子生物学
CiteScore
7.20
自引率
5.60%
发文量
132
审稿时长
1.7 months
期刊介绍:
Molecular Microbiology, the leading primary journal in the microbial sciences, publishes molecular studies of Bacteria, Archaea, eukaryotic microorganisms, and their viruses.
Research papers should lead to a deeper understanding of the molecular principles underlying basic physiological processes or mechanisms. Appropriate topics include gene expression and regulation, pathogenicity and virulence, physiology and metabolism, synthesis of macromolecules (proteins, nucleic acids, lipids, polysaccharides, etc), cell biology and subcellular organization, membrane biogenesis and function, traffic and transport, cell-cell communication and signalling pathways, evolution and gene transfer. Articles focused on host responses (cellular or immunological) to pathogens or on microbial ecology should be directed to our sister journals Cellular Microbiology and Environmental Microbiology, respectively.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


