Spatio-Chemical Deconvolution of the LiNi0.6Co0.2Mn0.2O2/Li6PS5Cl Interphase Layer in All-Solid-State Batteries Using Combined X-ray Spectroscopic Methods
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
The (electro)chemical degradation at the interface between Li6PS5Cl (LPSC) and LiNi0.6Co0.2Mn0.2O2 (NCM622) is systematically investigated using nondestructive synchrotron X-ray absorption spectroscopy and X-ray photoemission electron microscopy. These measurements were surface chemical depth profiling (from 2 to several hundred nanometers) and high-resolution elemental imaging of both LPSC and NCM622 particles. This analysis was complemented by galvanostatic cycling, impedance spectroscopy, and operando cell pressure characterization. Several correlations between interphase evolution and cell electrochemical performance are clarified, while some inconsistencies are rationalized and discussed. First, the intrinsic LPSC electrochemical oxidation mechanisms were studied using an LPSC:C65 working electrode (WE). The results showed that increased cell resistance during the first charge stemmed from polysulfide byproducts and particle contact loss due to LPSC volume shrinkage at the interface. Second, when using an NCM622:LPSC WE, species, such as SO32–, SO42–, and PO43–, were detected on both LPSC and NCM622 particles, while electrochemically inactive reduced transition metals were observed only at NCM622 surfaces. These species, initially present at open-circuit potential, increased after the first charge due to the chemical reactions between LPSC and NCM622 surface lattice oxygen. The estimated interphase thickness on the LPSC and NCM622 surfaces over the cycling remains below ∼3 nm. Our findings highlight that the formation of an electrochemically inactive NCM622 surface is a primary cause of impedance rise during the first charge, along with the formation of LPSC byproducts and contact loss. However, the continuous increase in cell resistance could not be attributed to further interphase growth after the first charge. We hypothesize that this may result from slow and progressive LPSC polymerization reactions (e.g., Li2P2S6 and P2S5) and structural changes at the NCM622 surface.
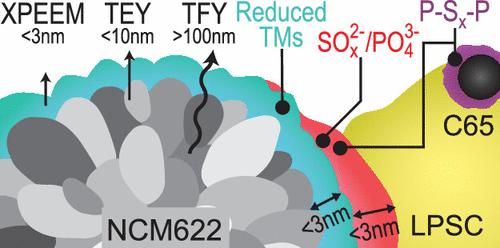
全固态电池LiNi0.6Co0.2Mn0.2O2/Li6PS5Cl间相层的空间化学反褶积
利用无损同步辐射 X 射线吸收光谱和 X 射线光电发射电子显微镜,系统地研究了 Li6PS5Cl(LPSC)和 LiNi0.6Co0.2Mn0.2O2(NCM622)之间界面的(电)化学降解。这些测量包括 LPSC 和 NCM622 颗粒的表面化学深度剖析(从 2 纳米到几百纳米)和高分辨率元素成像。电静力循环、阻抗光谱和操作室压力表征对上述分析进行了补充。澄清了相间演化与电池电化学性能之间的一些相关性,同时对一些不一致之处进行了合理的解释和讨论。首先,使用 LPSC:C65 工作电极(WE)研究了 LPSC 的内在电化学氧化机制。结果表明,第一次充电期间电池电阻的增加源于多硫化物副产品和 LPSC 在界面处体积收缩导致的颗粒接触损失。其次,在使用 NCM622:LPSC WE 时,LPSC 和 NCM622 颗粒上都检测到了 SO32-、SO42- 和 PO43- 等物种,而只有在 NCM622 表面才观察到电化学不活跃的还原过渡金属。由于 LPSC 和 NCM622 表面晶格氧之间的化学反应,这些最初存在于开路电位的物种在第一次充电后有所增加。在循环过程中,LPSC 和 NCM622 表面的估计相间厚度保持在 3 纳米以下。我们的研究结果突出表明,电化学不活泼的 NCM622 表面的形成是导致首次充电期间阻抗上升的主要原因,同时还形成了 LPSC 副产物和接触损失。然而,细胞电阻的持续增加不能归因于首次充电后相间的进一步生长。我们推测,这可能是缓慢渐进的 LPSC 聚合反应(如 Li2P2S6 和 P2S5)和 NCM622 表面结构变化的结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


