Design and biochemical evaluation of 2-cyclopropyl-thioureidobenzamide (CP-TBA) derivatives as potent HBV capsid assembly modulators targeting a novel binding site
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
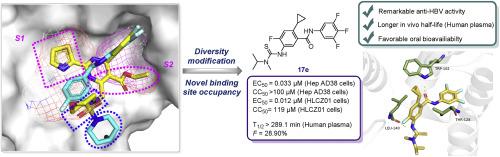
Hepatitis B virus (HBV) capsid assembly modulators (CAMs) represent a promising therapeutic approach in the treatment of chronic HBV infection. In the quest for effective therapeutics against chronic Hepatitis B virus (HBV) infection, we employed a novel binding site occupancy strategy to develop novel 2-cyclopropyl-thioureidobenzamide (CP-TBA) derivatives as potent HBV capsid assembly modulators (CAMs). Our diversity modification approach led to the identification of compound 17e, which demonstrated remarkable anti-HBV activity with an EC50 of 0.033 μM in HepAD38 cells. Molecular insights obtained through docking and dynamics simulations have provided a comprehensive understanding of the hydrogen bonding interactions between 17e and crucial residues of the HBV core protein, while also revealing the occupation of a novel binding site by the cyclopropyl group, thereby elucidating its inhibitory mechanism. Although 17e exhibited robust metabolic stability in plasma, it underwent rapid metabolism in human liver microsomes. This study underscores the potential of CP-TBA derivatives in crafting the next generation of HBV CAMs with enhanced activity and druggability.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


