Electron and proton storage on separate Ru and BaO domains mediated by conductive low-work-function carbon to accelerate ammonia synthesis
IF 42.8
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Ammonia (NH3) has gained attention as a carbon-free fuel and hydrogen carrier, making its energy-efficient production increasingly important. Here we demonstrate that Ru and BaO, connected by conductive carbon, can separately store e− and H+, like a chemical capacitor under NH3 synthesis conditions. H atoms generated on the Ru surface by H2 activation polarize into H+/e− pairs. Subsequently, H+ migrates over the carbon surfaces to neutralize basic BaO, while e− accumulates in conductive Ru/carbon. As the work function of carbon decreases, Ru gradually becomes enriched with e−, facilitating N2 activation via π-backdonation and alleviating H2 poisoning. Thus, an optimized catalyst synthesized using N-doped MWNT with the lowest work function, exhibited 7.4 times higher activity than a reference Ba–Ru/MgO catalyst. The results show that charge distribution within catalysts can be markedly altered under reaction conditions, and its rational control can enable the design of active NH3 synthesis catalysts. Ru and Ba are common partners within ammonia synthesis catalysts, but the mechanism by which the base promotes the metal is not fully understood. Here the use of conductive carbon supports reveals intriguing mechanistic aspects of this promotion effect and enables the generation of an advanced ammonia synthesis catalyst.

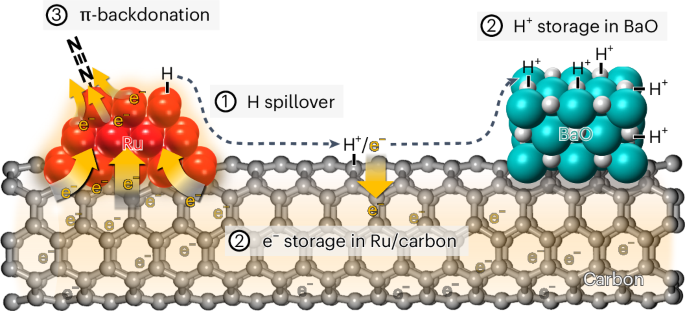
以导电低功耗碳为媒介,在独立的 Ru 和 BaO 域上存储电子和质子,加速氨的合成
氨(NH3)作为一种无碳燃料和氢载体已引起人们的关注,其节能生产日益重要。在这里,我们证明了Ru和BaO通过导电碳连接,可以在NH3合成条件下像化学电容器一样分别存储e -和H+。H2活化在Ru表面生成的H原子极化成H+/e−对。随后,H+在碳表面迁移以中和碱性BaO,而e−在导电Ru/碳中积累。随着碳的功函数减小,Ru逐渐富集e−,有利于π-反捐赠活化N2,缓解H2中毒。因此,使用n掺杂MWNT合成的优化催化剂具有最低的功函数,其活性比参考的Ba-Ru /MgO催化剂高7.4倍。结果表明,在不同的反应条件下,催化剂内部电荷分布会发生明显的变化,合理控制电荷分布可以设计出活性NH3合成催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


