Regioselectivity Switching in CYP107Pdh-Catalyzed VD3 Hydroxylation: A Structure-Guided Approach To Improve Calcidiol Production
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
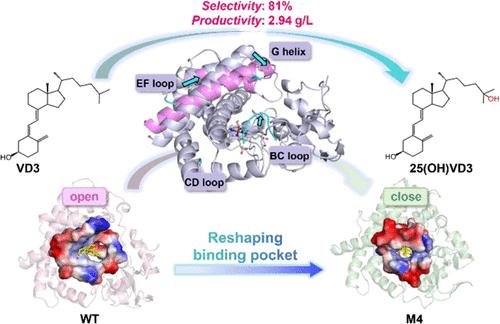
The biocatalytic production of 25-hydroxyvitamin D3 (25(OH)VD3, calcidiol) represents a superior alternative to traditional chemical synthesis. However, the reported VD3 hydroxylases generally exhibit suboptimal catalytic efficiency, limiting their practical applications. In this study, a cytochrome P450 CYP107Pdh from Pseudonocardia dioxanivorans_CB1190, which displays unique C26 hydroxylation activity on VD3, was identified. Then, structure-guided loop engineering combined with binding pocket reshaping was conducted on CYP107Pdh, leading to the generation of the quintuple variant 89_90insIP/T112A/V161L/G186V (M4). This variant shifted the regioselectivity from 91% C26 in the wild type (WT) to 81% C25 for calcidiol production. In addition, variant M4 showed a remarkable enhancement in catalytic activity, achieving a catalytic efficiency (kcat/Km) that is 75-fold higher than that of the WT. Computational analyses revealed that the regioselectivity shift and activity improvement are primarily attributed to a conformational transition in the substrate-binding pocket from an open to a more closed state, which optimizes substrate binding and facilitates efficient 25-hydroxylation of VD3. Finally, a semipreparative biotransformation yielded 2.64 g of crystalline calcidiol (95% purity) from a 1-L reaction, thereby expanding the enzyme library of VD3 hydroxylases and underscoring its potential for industrial-scale production of calcidiol.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


