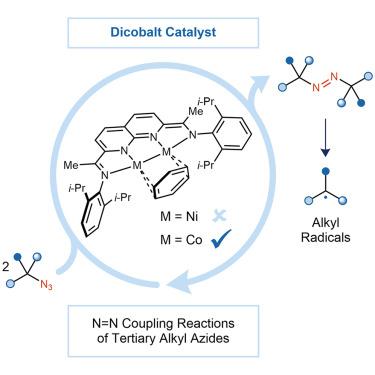
Dicobalt-catalyzed N=N coupling reactions of tertiary alkyl azides to form azoalkanes
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Azoalkanes can serve as radical precursors for various catalytic and stoichiometric C–C bond-forming reactions. However, their use in these processes is hampered by the complexity of their synthesis, which often requires multiple steps and strong oxidants. Here, we report a direct denitrogenative dimerization of tertiary alkyl azides to form azoalkanes. The reaction uses a dicobalt catalyst, which is uniquely effective in this transformation relative to analogous monocobalt catalysts and an isostructural dinickel catalyst. Critical to the N=N coupling reactivity is the formation of a dicobalt imido intermediate that is resistant to undergoing competing H-atom abstraction. The catalytic N=N coupling provides access to a broad scope of tertiary azoalkanes, and the resulting products can be used to form hindered C–C bonds between quaternary carbons.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


