Endoplasmic reticulum stress as a driver and therapeutic target for kidney disease
IF 39.8
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
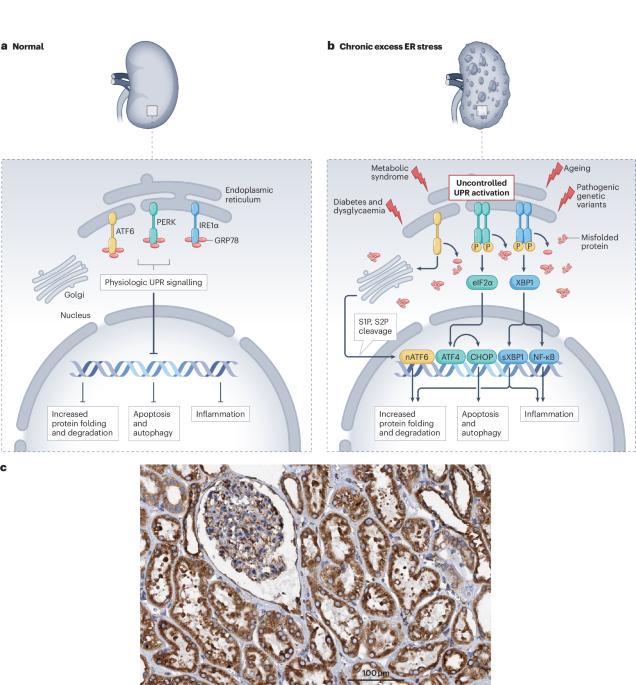
The endoplasmic reticulum (ER) has crucial roles in metabolically active cells, including protein translation, protein folding and quality control, lipid biosynthesis, and calcium homeostasis. Adverse metabolic conditions or pathogenic genetic variants that cause misfolding and accumulation of proteins within the ER of kidney cells initiate an injurious process known as ER stress that contributes to kidney disease and its cardiovascular complications. Initiation of ER stress activates the unfolded protein response (UPR), a cellular defence mechanism that functions to restore ER homeostasis. However, severe or chronic ER stress rewires the UPR to activate deleterious pathways that exacerbate inflammation, apoptosis and fibrosis, resulting in kidney injury. This insidious crosstalk between ER stress, UPR activation, oxidative stress and inflammation forms a vicious cycle that drives kidney disease and vascular damage. Furthermore, genetic variants that disrupt protein-folding mechanisms trigger ER stress, as evidenced in autosomal-dominant tubulointerstitial kidney disease and Fabry disease. Emerging therapeutic strategies that enhance protein-folding capacity and reduce the burden of ER stress have shown promising results in kidney diseases. Thus, integrating knowledge of how genetic variants cause protein misfolding and ER stress into clinical practice will enhance treatment strategies and potentially improve outcomes for various kidney diseases and their vascular complications. Endoplasmic reticulum (ER) stress is known to exacerbate chronic kidney disease and cardiovascular disease. Here, the authors discuss the role of ER stress in kidney disease and the link between ER stress, chronic kidney disease and cardiovascular disease.
内质网应激是肾脏疾病的驱动因素和治疗靶点
内质网(ER)在新陈代谢活跃的细胞中起着至关重要的作用,包括蛋白质翻译、蛋白质折叠和质量控制、脂质生物合成和钙平衡。不良的新陈代谢条件或致病基因变异会导致肾细胞ER内蛋白质的错误折叠和积累,从而引发一种称为ER应激的损伤过程,导致肾脏疾病及其心血管并发症。启动ER应激会激活未折叠蛋白反应(UPR),这是一种细胞防御机制,其功能是恢复ER平衡。然而,严重或慢性的ER应激会重新连接UPR,激活有害途径,加剧炎症、细胞凋亡和纤维化,导致肾脏损伤。ER应激、UPR激活、氧化应激和炎症之间的这种隐秘串联形成了一个恶性循环,导致肾脏疾病和血管损伤。此外,破坏蛋白质折叠机制的基因变异会引发ER应激,常染色体显性肾小管间质性肾病和法布里病就是证明。增强蛋白质折叠能力和减轻ER应激负担的新兴治疗策略在肾脏疾病中显示出良好的效果。因此,将基因变异如何导致蛋白质错误折叠和ER应激的知识融入临床实践,将加强治疗策略,并有可能改善各种肾脏疾病及其血管并发症的治疗效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Nephrology
医学-泌尿学与肾脏学
CiteScore
39.00
自引率
1.20%
发文量
127
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Nephrology aims to be the premier source of reviews and commentaries for the scientific communities it serves.
It strives to publish authoritative, accessible articles.
Articles are enhanced with clearly understandable figures, tables, and other display items.
Nature Reviews Nephrology publishes Research Highlights, News & Views, Comments, Reviews, Perspectives, and Consensus Statements.
The content is relevant to nephrologists and basic science researchers.
The broad scope of the journal ensures that the work reaches the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


