Light-activated hypervalent iodine agents enable diverse aliphatic C–H functionalization
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The functionalization of aliphatic C–H bonds is a crucial step in the synthesis and transformation of complex molecules relevant to medicinal, agricultural and materials chemistry. As such, there is substantial interest in the development of general synthetic platforms that enable the efficient diversification of aliphatic C–H bonds. Here we report a hypervalent iodine reagent that releases a potent hydrogen atom abstractor for C–H activation under mild photochemical conditions. Using this reagent, we demonstrate selective (N-phenyltetrazole)thiolation of aliphatic C–H bonds for a broad scope of substrates. The synthetic utility of the thiolated products is showcased through various derivatizations. Simply by altering the radical trapping agent, our method can directly transform C–H bonds into diverse functionalities, including C–S, C–Cl, C–Br, C–I, C–O, C–N, C–C and C=C bonds. Aliphatic C–H functionalization is a valuable tool in organic synthesis. Now a hypervalent iodine reagent has been shown to release a potent hydrogen atom abstractor for C–H activation under mild photochemical conditions. This enables the transformation of C–H bonds into diverse functional groups with tunable control over the site selectivity.

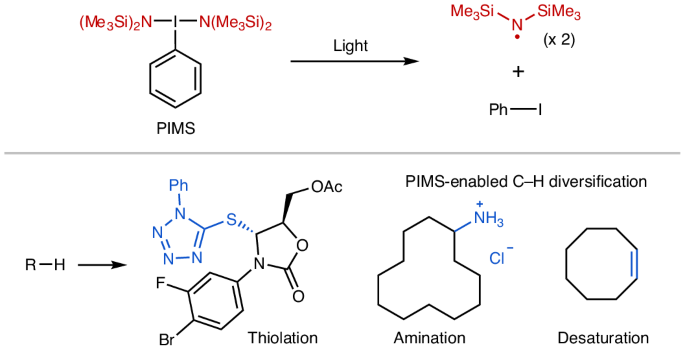
光活化的高价碘剂使多种脂肪C-H功能化
脂肪族C-H键的功能化是合成和转化与医药、农业和材料化学相关的复杂分子的关键步骤。因此,人们对开发能够使脂肪族C-H键有效多样化的通用合成平台非常感兴趣。在这里,我们报道了一种高价碘试剂,它在温和的光化学条件下释放出一种有效的氢原子萃取剂,用于C-H活化。使用该试剂,我们证明了广泛底物的脂肪族C-H键的选择性(n -苯基四唑)硫基化。通过各种衍生化,展示了硫代化产物的合成效用。通过改变自由基捕获剂,我们的方法可以直接将C - h键转化为多种官能团,包括C - s、C - cl、C - br、C - i、C - o、C - n、C - C和C=C键。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


