Nanocomposite beads with engineered pore architecture for improved removal of emerging contaminants from water
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
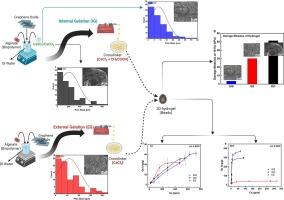
The rapid crosslinking process for the formation of biopolymeric beads typically limits their adsorption rates and capacities due to suboptimal pore structures and morphology. In conventional beads, the highly porous core is enclosed by a semi-permeable shell, restricting mass transfer. To address this, we engineered the pore architecture of the beads using an internal gelation (IG) process. This approach overcomes diffusion resistance barriers, and improves the environmental performance of the beads. By controlling the gelation process with CaCO3 (IGC), which is a gas forming agent, and CaSO4 (IGS), which controls the rate of cross-linking from within the beads, we tailored the pore architecture. We characterized the beads in terms of elemental composition, morphology, mechanical properties, and stability. The IG beads, containing only 3.5 wt% graphene oxide (GO), showed significantly faster adsorption rates—up to two orders of magnitude higher for diclofenac and twice as fast for tetracycline removal compared to externally gelled (EG) beads. IGS beads demonstrated superior adsorption capacities, with multiple-fold increases for both diclofenac and tetracycline, due to enhanced intraparticle diffusion enabled by the IG process. The exceptional performance of IGS beads is attributed to the creation of a highly porous outer shell with hierarchical linkage to the inner core, reducing diffusion resistance and maximizing the adsorption potential of GO’s sp2 hybridized domains, without the need for chemical reduction of the nanosheets. IGC beads, with improved mechanical properties, also showed remarkable adsorption capacities comparable to IGS beads, and surpassing that of EG beads. Our technique, not material specific, can be used in any hydrogel that is made by ionic cross-linking for a broad range of applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


