Boosting the reduction of CO2 and dimethylamine for C–N bonding to synthesize DMF via modulating the electronic structures of indium single atoms
IF 32.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
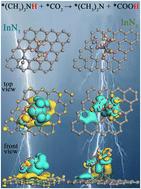
The electrocatalytic reduction of CO2 and dimethylamine (HN(CH3)2) for C–N coupling is a promising strategy for synthesizing N,N-dimethylformamide (DMF). However, the generation of suitable coupling intermediates via the dehydrogenation of HN(CH3)2 and reduction hydrogenation of CO2 is the key challenge for achieving C–N bonding to synthesize DMF. We optimized the electronic structure of HN(CH3)2 and CO2 for adsorption on indium single atoms by adjusting the coordination structure, thereby promoting the hydrogen transfer from nitrogen of dimethylamine to CO2, which generated the intermediates of *N(CH3)2 and *COOH for C–N bonding to synthesize DMF. The yield of DMF synthesized on InN3 reached 41.3 μmol L−1 h−1, which was about 12 times greater than that of InN4 at −0.8 V. In situ technology and DFT calculations jointly demonstrated that compared with InN4, InN3 optimized the electron distribution of adsorbed CO2 and HN(CH3)2. The electron density of hydrogen on HN(CH3)2 decreased, exhibiting its electrophilic properties. In addition, oxygen of CO2 accumulated electrons near the dimethylamine end and exhibited strong electron-rich properties, which led to hydrogen transfer from dimethylamine to CO2, generating the species *N(CH3)2 and *COOH that are conducive to C–N coupling to synthesize DMF on InN3. This work provides important theoretical guidance for the C–N coupling of CO2 and amines.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Energy & Environmental Science
化学-工程:化工
CiteScore
50.50
自引率
2.20%
发文量
349
审稿时长
2.2 months
期刊介绍:
Energy & Environmental Science, a peer-reviewed scientific journal, publishes original research and review articles covering interdisciplinary topics in the (bio)chemical and (bio)physical sciences, as well as chemical engineering disciplines. Published monthly by the Royal Society of Chemistry (RSC), a not-for-profit publisher, Energy & Environmental Science is recognized as a leading journal. It boasts an impressive impact factor of 8.500 as of 2009, ranking 8th among 140 journals in the category "Chemistry, Multidisciplinary," second among 71 journals in "Energy & Fuels," second among 128 journals in "Engineering, Chemical," and first among 181 scientific journals in "Environmental Sciences."
Energy & Environmental Science publishes various types of articles, including Research Papers (original scientific work), Review Articles, Perspectives, and Minireviews (feature review-type articles of broad interest), Communications (original scientific work of an urgent nature), Opinions (personal, often speculative viewpoints or hypotheses on current topics), and Analysis Articles (in-depth examination of energy-related issues).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


