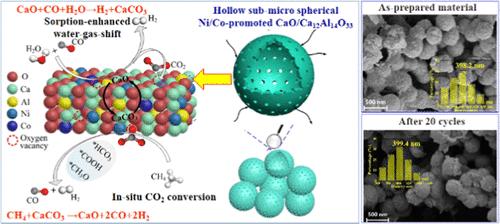
Hollow Submicrospherical Ni/Co-Promoted CaO/Ca12Al14O33 for H2 Production from Sorption-Enhanced Water–Gas Shift with In Situ CO2 Conversion via CH4 Reforming of CaCO3
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
CaO sorbent/catalyst bifunctional materials are promising for CO2 capture in sorption-enhanced H2 production such as sorption-enhanced water–gas shift. For simultaneous H2 production with CO2 in situ capture and utilization, the integrated process of sorption-enhanced water–gas shift and in situ CO2 conversion by CH4 reforming of CaCO3 was proposed. This work focused on the tailored design of a CaO sorbent/catalyst bifunctional material for both efficient H2 production and in situ CO2 conversion in this integrated process. The template-assisted strategy of hydrothermal carbonization followed by self-reduction and template removal via steam gasification was first proposed to obtain the hollow submicrospherical Ni/Co-promoted CaO/Ca12Al14O33. The as-synthesized material exhibits high and stable H2 production, CO2 capture, and in situ CO2 conversion performance in the integrated process due to the unique hollow submicrospherical structure and enhanced catalytic activity. Ni–Co interaction boosts oxygen vacancy and Ni–Co alloy, which are the active catalytic sites for the water–gas shift and CH4–CaCO3 reactions. Moreover, the oxygen vacancy-mediated mechanism on CH4 reforming of CaCO3 over the hollow submicrospherical Ni/Co-promoted CaO/Ca12Al14O33 is confirmed. After 20 cycles, CO conversion from sorption-enhanced water–gas shift using the as-synthesized material retains 97.0%, accompanied by high CH4 conversion of 95.1% and the H2/CO molar ratio close to unity from CH4 reforming of CaCO3.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


