A systematic implementation of padlock probing-based rolling circle amplification in an integrated microfluidic device for quantitative biomolecular analyses
IF 5.7
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Background
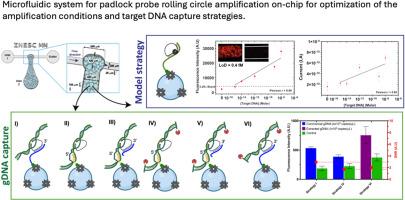
Pathogen detection in primary care is crucial not only to identify viruses like SARS-CoV-2 but also for antibiotic-resistant bacteria. While microfluidic devices enable point-of-care diagnostics, they often lack sufficient sensitivity. On-chip isothermal amplification techniques, such as padlock probing-based rolling circle amplification (PLP-RCA), can enhance specificity and sensitivity while keeping device complexity low. However, integrating PLP-RCA on-chip requires precise optimization of enzyme concentrations, flow conditions, and target capture to achieve its full potential.Results
This study demonstrates a microfluidic RCA assay using porous agarose microbeads as a solid-phase capture, packed inside a microfluidic device. Various target capture strategies were systematically compared and quantitatively investigated, progressing from single-stranded synthetic DNA oligonucleotides to double-stranded Staphylococcus aureus genomic DNA. The best strategy for double-stranded Staphylococcus aureus genomic DNA used a primer bound to the beads that capture the PLP and the target genomic DNA. The system integrates an amorphous-hydrogenated silicon (a-Si:H) thin film p-i-n photodiode and a high-pass interference filter, enabling on-chip fluorescence signal acquisition of amplicons. This integration allows for a fully functional PLP-RCA assay on-chip, along with the added merits of device portability and compatibility with clinical demands.Significance and Novelty
This study systematically evaluates single- and double-stranded target capture for on-chip PLP-RCA assays. It demonstrates the successful integration of microfluidics with a solid-phase capture medium and fluorescence detection system. The findings highlight the potential of this platform for developing sensitive, portable pathogen detection devices suited for clinical applications.”
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


