Surface sulfur functionalized defects on the synergistic and competitive effects of CO2 and H2O adsorption: Density functional theory study
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Heteroatom doping can significantly enhance the CO2 adsorption capacity of carbon-based materials, but it also increases the hydrophilicity of the carbon matrix. This can lead to the competitive adsorption of CO2 and H2O becoming more pronounced in humid environments. In this study, the co-adsorption behaviors of H2O and CO2 on carbon surfaces modified with various sulfur functional groups were investigated at the molecular level using density functional theory. The adsorption mechanisms were comprehensively analyzed through IGMH, QTAIM, EDA-FF, and charge transfer analysis. ESP analysis revealed that the absolute values of the local maxima and minima of the water molecules’ electrostatic potential were higher than those of CO2, indicating that water molecules exhibit greater adsorption stability and hydrogen bonding capability on highly polar functional groups. QTAIM analysis identified the specific interaction pathways and strengths between the atoms of CO2 and H2O, while IGMH analysis showed that the interactions between CO2, H2O, and the porous carbon are primarily weak van der Waals forces. EDA-FF results indicate that electrostatic and dispersive interactions are the dominant forces in the co-adsorption of CO2 and H2O. The enhanced adsorption stability of CO2 in the co-adsorption system is mainly due to the additional unsaturated carbon sites created by basal defects, which significantly strengthen the van der Waals interactions of CO2. Additionally, hydrogen bonding between CO2 and H2O further promotes CO2 adsorption energy. This study underscores the critical role of sulfur functionalization in modulating adsorption behavior on carbon surfaces and provides valuable theoretical insights for designing advanced adsorbent materials capable of synergistically adsorbing multi-component gases.
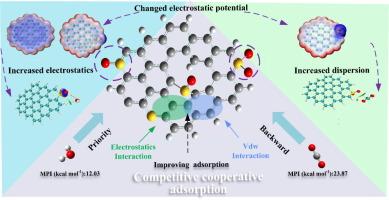
掺杂杂原子可显著提高碳基材料的二氧化碳吸附能力,但同时也会增加碳基质的亲水性。这可能导致二氧化碳和水的竞争吸附在潮湿环境中变得更加明显。本研究采用密度泛函理论,在分子水平上研究了经各种硫官能团修饰的碳表面对 H2O 和 CO2 的共吸附行为。通过 IGMH、QTAIM、EDA-FF 和电荷转移分析全面分析了吸附机理。ESP 分析表明,水分子静电势的局部最大值和最小值的绝对值均高于 CO2,这表明水分子在高极性官能团上表现出更高的吸附稳定性和氢键能力。QTAIM 分析确定了 CO2 和 H2O 原子间的特定相互作用途径和强度,而 IGMH 分析表明 CO2、H2O 和多孔碳之间的相互作用主要是弱范德华力。EDA-FF 结果表明,静电作用和分散作用是 CO2 和 H2O 共吸附的主要作用力。共吸附体系中 CO2 吸附稳定性增强的主要原因是基底缺陷产生了额外的不饱和碳位点,从而大大加强了 CO2 的范德华相互作用。此外,CO2 和 H2O 之间的氢键作用也进一步提高了 CO2 的吸附能。这项研究强调了硫功能化在调节碳表面吸附行为中的关键作用,并为设计能够协同吸附多组分气体的先进吸附材料提供了宝贵的理论见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


