Targeted Protein Degradation in Cancer Therapy via Hydrophobic Polymer-Tagged Nanoparticles
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
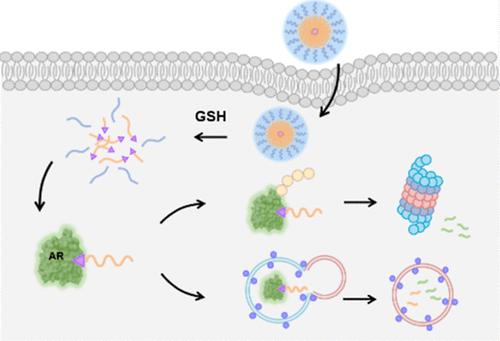
Targeted protein degradation (TPD) strategies offer a significant advantage over traditional small molecule inhibitors by selectively degrading disease-causing proteins. While small molecules can lead to recurrence and resistance due to compensatory pathway activation, TPD addresses this limitation by promoting protein degradation, thereby reducing the likelihood of recurrence and resistance over the long-term. Despite these benefits, bifunctional TPD molecules face challenges such as low solubility, poor bioavailability, and limited tumor specificity. In this study, we developed polymer-based nanoparticles that combine TPD strategies with nanotechnology through a hydrophobic tagging method. Hydrophobic polymer-tagged nanoparticles facilitate targeted protein degradation by incorporating hydrophobic polymers that mimic hydrophobic residues in misfolded proteins. This system combines degradation and delivery capabilities within a polymer-based platform, inducing protein degradation while improving solubility, stability, and tumor targeting. These nanoparticles consist of a block copolymer composed of an androgen receptor ligand (ARL)-conjugated hydrophobic polylactic acid (PLA) and a hydrophilic polyethylene glycol (PEG), connected by a GSH-cleavable disulfide bond. In aqueous solutions, this block copolymer (ARL-PLA-SS-PEG) forms micelles that degrade in reducible cellular environments. The micelles demonstrated significant in vitro degradation of the target androgen receptor (AR). Furthermore, they achieved substantial tumor accumulation and significantly inhibited tumor growth in a tumor-bearing mouse model. A mechanistic study revealed that the micelle-mediated TPD follows a dual pathway involving both proteasome and autophagosome. This approach has the potential to serve as a universal platform for protein degradation, eliminating the need to develop disease-specific TPD molecules.
通过疏水聚合物标记纳米颗粒靶向蛋白质降解癌症治疗
靶向蛋白降解(TPD)策略通过选择性降解致病蛋白,提供了优于传统小分子抑制剂的显著优势。由于代偿通路的激活,小分子可能导致复发和耐药,而TPD通过促进蛋白质降解来解决这一限制,从而降低长期复发和耐药的可能性。尽管有这些优点,双功能TPD分子仍面临溶解度低、生物利用度差和肿瘤特异性有限等挑战。在这项研究中,我们开发了基于聚合物的纳米颗粒,通过疏水标记方法将TPD策略与纳米技术相结合。疏水聚合物标记的纳米颗粒通过掺入疏水聚合物来模拟错误折叠蛋白质中的疏水残基,从而促进靶向蛋白质降解。该系统结合了聚合物平台的降解和传递能力,诱导蛋白质降解,同时提高溶解度、稳定性和肿瘤靶向性。这些纳米颗粒由雄激素受体配体(ARL)偶联疏水聚乳酸(PLA)和亲水聚乙二醇(PEG)组成的嵌段共聚物组成,由gsh可切割的二硫键连接。在水溶液中,这种嵌段共聚物(ARL-PLA-SS-PEG)形成胶束,在可还原的细胞环境中降解。胶束对靶雄激素受体(AR)有显著的体外降解作用。此外,它们在荷瘤小鼠模型中实现了大量的肿瘤积累,并显著抑制了肿瘤的生长。一项机制研究表明,胶束介导的TPD遵循一个涉及蛋白酶体和自噬体的双重途径。这种方法有潜力作为蛋白质降解的通用平台,消除了开发疾病特异性TPD分子的需要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


