Steering the Electronic Microenvironment of Ruthenium Sites via Boron Buffering Enables Enhanced Hydrogen Evolution under a Universal pH Range
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Optimizing the microenvironment of active sites is crucial for enhancing the catalytic activity of the hydrogen evolution reaction (HER) across various pH conditions. Here, guided by theoretical predictions of boron (B)-doping’s electronic buffering effect on ruthenium (Ru) at the atomic scale, a highly efficient and universal-pH Ru-based HER electrocatalyst (Ru-NBC) by introducing B and nitrogen (N) into a carbon (C) matrix was designed. The Ru-NBC catalyst demonstrated exceptional HER activity, requiring overpotentials of 27, 40, and 68 mV in 1 M KOH, 0.5 M H2SO4, and 1 M phosphate buffer solution (PBS), respectively, to achieve a current density of 10 mA cm–2. In situ Raman spectroscopy, ambient-pressure X-ray photoelectron spectroscopy, and potential of zero charge measurements revealed that B-doping modulates the local Ru microenvironment, restructuring the distribution balance of the interfacial water hydrogen-bond network within the electrochemical double layer and thereby facilitating water adsorption and dissociation. Density functional theory calculations further verified that the electronic buffering effect of B optimizes hydrogen adsorption in acidic media and water activation in alkaline conditions, resultantly contributing to the universal-pH HER performance. This study could provide guidance for the design of advanced electrocatalysts through modulation of the local microenvironment of active sites for energy storage and conversion.
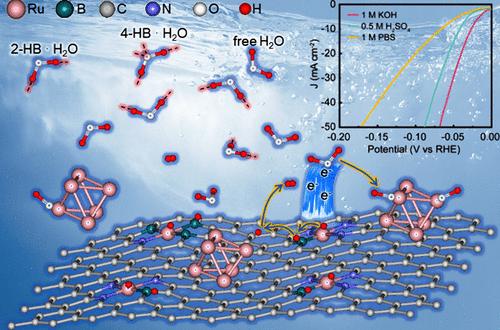
优化活性位点的微环境对于提高氢进化反应(HER)在不同 pH 值条件下的催化活性至关重要。本文以硼(B)掺杂在原子尺度上对钌(Ru)的电子缓冲作用的理论预测为指导,通过在碳(C)基体中引入硼和氮(N),设计出了一种高效、通用 pH 值的 Ru 基氢化反应电催化剂(Ru-NBC)。Ru-NBC 催化剂表现出卓越的 HER 活性,在 1 M KOH、0.5 M H2SO4 和 1 M 磷酸盐缓冲溶液 (PBS) 中分别需要 27、40 和 68 mV 的过电位才能达到 10 mA cm-2 的电流密度。原位拉曼光谱、常压 X 射线光电子能谱和零电荷电位测量结果表明,B 掺杂改变了局部 Ru 的微环境,调整了电化学双层内界面水氢键网络的分布平衡,从而促进了水的吸附和解离。密度泛函理论计算进一步验证了 B 的电子缓冲效应可优化酸性介质中的氢吸附和碱性条件下的水活化,从而有助于提高通用 PH HER 性能。这项研究可为通过调节活性位点的局部微环境来设计先进的电催化剂提供指导,从而实现能量存储和转换。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


