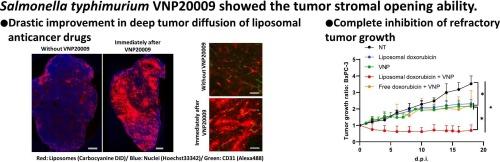
Tumor-stromal opening via S. typhimurium VNP20009 administration for complete inhibition of refractory tumor growth with liposomal anticancer drugs
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Many clinical tumors exhibit a vascular endothelium covered by mural cells and stroma with abundant collagen fibers, which greatly inhibit the penetration of nanoparticle drug delivery systems (DDS) formulations deep into the tumors. We previously found that Salmonella typhimurium VNP20009 attracting attention as live bacterial therapeutics, which is a novel pharmaceutical modality for cancer treatment, can grow within deep tumors with abundant stroma and tight vasculature. Because this finding interestingly indicates that VNP20009 administration disrupts vascular and stromal structures even in refractory tumors, we investigated the possibility that VNP20009 administration improves DDS formulations migrations into tumors in this study. VNP20009 co-administration drastically improved the translocation and diffusion of liposomes deep into the tumors, particularly in stroma-rich xenografted tumors, indicating its tumor stromal opening ability. Furthermore, this approach can completely inhibit tumors in various refractory tumor models, including pancreatic cancers, using liposomal doxorubicin (Doxil®) and liposomal irinotecan (Onivyde®). Notably, this remarkable anticancer effect is not simply attributed to the therapeutic effects of liposomal anticancer drugs and VNP20009, but it involves an additional effect, improving the intratumor pharmacokinetics of liposomal anticancer drugs following VNP20009 co-administration. The unique tumor stromal opening ability of VNP20009 demonstrated in this study is a promising strategy for resolving the major challenges faced by tumor DDS.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


