Variable dual C-Cl isotope slopes of trichloromethane transformation by alkaline-activated persulfate under different simulated field conditions
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
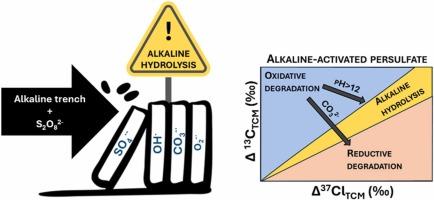
Laboratory experiments were conducted to evaluate the potential of δ13C and δ37Cl isotopic values of trichloromethane (TCM) to monitor and quantify its transformation during alkaline persulfate (PS) activation. Batch experiments were designed to replicate different TCM:PS molar ratios, pH values, the presence of CO32- ion and the simulation of an alkaline interception trench. Results revealed three distinct C-Cl isotopic trends; First, despite differences in degradation kinetics, isotopic trends were consistent across TCM:PS molar ratios (ΛC-Cl between 23 ± 10 and 33 ± 6), suggesting that radical activation remained unaffected. Conversely, at pH 12.8, alkaline hydrolysis (AH) became the predominant degradation process (ΛC-Cl of 9 ± 1 and 11 ± 1) over reaction with PS derived radical species. Finally, in the presence of excess CO32- ion, which acts as radical scavenger probably affecting the radical species involved in TCM degradation, a ΛC-Cl value of 5.5 ± 0.6 was observed, suggesting a reductive degradation reaction. Therefore, our results reveal, for the first time, that the dual C-Cl isotope slope during TCM degradation by PS varies significantly depending on field conditions. The unexpected accumulation of higher chlorinated byproducts, such as hexachloroethane, during TCM degradation by alkaline-activated PS was observed for the first time and further research is needed in real open-systems to assess its potential environmental implications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


