Removal of nickel ions from industrial wastewater using tms-EDTA-functionalized Ti3C2Tx: Experimental and statistical physics modeling
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
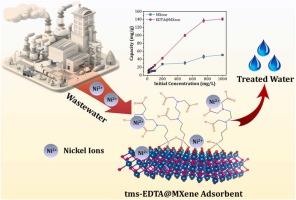
This study investigates the surface modification of Ti3C2Tx MXene using tms-EDTA (EDTA@MXene) to develop an efficient adsorbent for divalent heavy metal cations, such as Cd²⁺, Cu²⁺, Ni²⁺, Pb²⁺, and Zn²⁺, from contaminated water. EDTA@MXene showed significantly enhanced adsorption capacities for these ions compared to pristine MXene. Using nickel ion (Ni²⁺) as a model adsorbate, EDTA@MXene demonstrated remarkable removal efficiency, reaching a maximum adsorption capacity of 249.5 mg/g as compared to the 61.4 mg/g of pristine MXene with fast kinetics and attaining equilibrium within 30 minutes. The results indicated that Ni²⁺ adsorption followed a pseudo-second-order kinetic model, with equilibrium data fitting both Langmuir and Freundlich isotherm models. As the classical adsorption models remained inconclusive on the underlying adsorption mechanisms, advanced statistical physics models were subsequently applied for deeper investigation. The findings revealed that Ni²⁺ ions adsorbed onto the surface in a non-parallel orientation. The adsorption process was reversible, endothermic, and driven mainly by physical interactions, with higher temperatures favoring greater adsorption capacity. EDTA@MXene demonstrated excellent reusability, maintaining high (>80%) regeneration efficiency after five regeneration cycles. It also exhibited a high adsorption capacity for Ni²⁺ ions from nickel electroplating wastewater, highlighting its potential for real application in the treatment of metal-contaminated industrial wastewater.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


