Elucidating the Influence of N Doping on Production of ε-Caprolactone Catalyzed by Nanocarbon: A Kinetic Model from Batch to Semibatch and Continuous-Flow Operations
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Nanocarbons have been identified as effective catalysts to promote the Baeyer–Villiger (BV) oxidation of cyclohexanone (Cy═O) toward ε-caprolactone (ε-CL). To effectively enlarge the production capacity of ε-CL, a kinetic model with simplified two main reactions (BV oxidation and aldehyde self-oxidation) catalyzed by carbon nanotubes (CNTs) and N-doped CNTs was established. The enhancement of N dopants was observed to facilitate the rate constant (k) and lower the energy barrier (Ea). Meanwhile, the negative value of reaction order for the BV oxidation was obtained, implying competitive adsorption between ketone and aldehyde on the carbon surface, as confirmed by the density functional theory simulations. Also, the high ratio of ketone/aldehyde favored the formation of ε-CL and improved the aldehyde efficiency (η). Based on the established kinetic model in the batch reaction, the semibatch and continuous-flow reactions were performed, validating the rationality and feasibility of the reaction model.
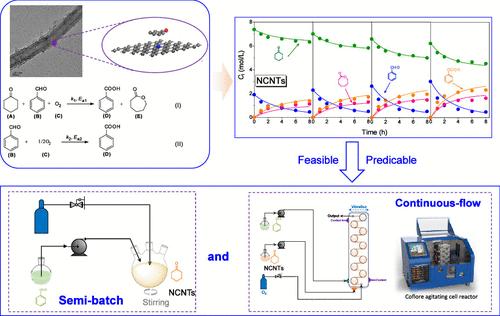
N掺杂对纳米碳催化生产ε-己内酯的影响:间歇-半间歇连续流动力学模型
纳米碳被认为是促进环己酮(Cy═O)向ε-己内酯(ε-CL)的拜尔-维里格(BV)氧化反应的有效催化剂。为了有效提高ε-CL 的生产能力,建立了一个简化的由碳纳米管(CNTs)和掺杂 N 的 CNTs 催化的两个主要反应(BV 氧化和醛自氧化)的动力学模型。观察到掺杂 N 的增强促进了速率常数(k)的提高并降低了能垒(Ea)。同时,密度泛函理论模拟证实,BV 氧化反应阶次为负值,这意味着酮和醛在碳表面的吸附具有竞争性。同时,高比例的酮/醛有利于ε-CL 的形成,并提高了醛效率(η)。根据已建立的间歇反应动力学模型,进行了半间歇反应和连续流动反应,验证了反应模型的合理性和可行性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


