Clinical Trial: A Phase 2b Study to Evaluate the Efficacy and Safety of MK-3655 in Individuals With Pre-Cirrhotic MASH
Abstract
Background
Fibroblast growth factor 21 (FGF21) is a metabolic regulator with demonstrated efficacy for the treatment of metabolic dysfunction-associated steatohepatitis (MASH). FGF21 signals through ‘c’ isoforms of the FGF receptors (FGFR) 1–3 and the co-receptor β-klotho.
Aims
We report the safety and efficacy of MK-3655, a monoclonal antibody that binds β-klotho and selectively activates the FGFR1c/β-klotho co-receptor complex, in patients with pre-cirrhotic MASH.
Methods
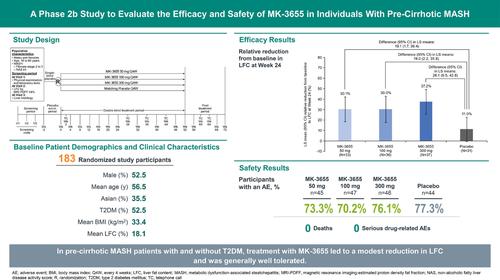
Phase 2b, randomised, multicenter, double-blind, placebo-controlled, parallel-group study in patients with pre-cirrhotic MASH (NAS ≥ 4 and MASH CRN fibrosis score Stage 2 or 3). Participants were randomised 1:1:1:1 to receive MK-3655 50 mg, 100 mg, 300 mg, or matching placebo subcutaneously every 4 weeks. The primary endpoint was MASH resolution without worsening of fibrosis by histology at Week 52. An interim analysis (IA) of liver fat content (LFC) was planned once ≥ 25 participants per treatment group completed an MRI-PDFF assessment at Week 24.
Results
Among 183 participants, mean BMI was 33.4 kg/m2, mean LFC was 18.1%, and 52.5% had type 2 diabetes. At the IA, the differences from placebo in relative reduction from baseline in LFC were assessed as insufficient for continuation of the trial. Among participants with Week 24 LFC assessment, percent relative reductions from baseline (LS mean difference vs. placebo) for MK-3655 50 mg (N = 33), 100 mg (N = 36), and 300 mg (N = 31), were 19.1%, 19.0%, and 26.1%, respectively. MK-3655 was generally well tolerated.
Conclusions
In patients with pre-cirrhotic MASH, treatment with MK-3655 resulted in a modest reduction in LFC at 24 weeks.
Clinical Trial Number
EudraCT: 2019-003048-63; NCT: 04583423.


 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


