Stereospecific access to α-haloalkyl esters via enol ester epoxides and synthesis of a C3–C21 fragment of bastimolide A†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We report a 14-step synthesis of a C3–C21 fragment of bastimolides A and B, antimalarial macrocyclic polyketides. A crucial ring-opening reaction of an enol ester epoxide showed previously unexplored reactivity, leading to an asymmetric synthesis of α-haloalkyl esters. The α-haloalkyl ester synthesis was shown to be stereospecific, and provided access to a key α-silyloxyaldehyde to initiate application of configuration-encoded 1,5-polyol synthesis. This strategy established the C11/C15 and C15/C19 remote stereochemical relationships of the bastimolides. The potential of this C3–C21 fragment for coupling to C22–C41 was established using a Mukaiyama aldol reaction with a simple enolsilane.
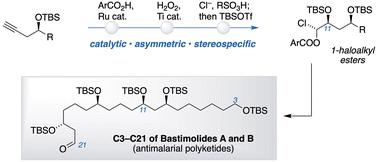
烯醇酯环氧化物对α-卤代烷基酯的立体定向接触及basbasolide a C3-C21片段的合成。
我们报道了14步合成抗疟疾大环多酮basbasolides a和B的C3-C21片段。烯醇酯环氧化物的开环反应显示出前所未有的反应活性,导致α-卤代烷基酯的不对称合成。α-卤代烷基酯的合成具有立体特异性,并为α-硅氧基醛的合成提供了通道,从而开启了构型编码1,5-多元醇合成的应用。该策略建立了C11/C15和C15/C19的远程立体化学关系。用简单烯醇硅烷与Mukaiyama醛醇反应确定了该C3-C21片段与C22-C41偶联的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


