Mitochondrial translation regulates terminal erythroid differentiation by maintaining iron homeostasis
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
Science Advances
Pub Date : 2025-02-21
引用次数: 0
Abstract
Mitochondrial tRNA taurine modifications mediated by mitochondrial tRNA translation optimization 1 (Mto1) is essential for the mitochondrial protein translation. Mto1 deficiency was shown to induce proteostress in embryonic stem cells. A recent finding that a patient with MTO1 gene mutation showed severe anemia led us to hypothesize that Mto1 dysfunctions may result in defective erythropoiesis. Hematopoietic-specific Mto1 conditional knockout (cKO) mice were embryonic lethal and showed niche-independent defect in erythroblast proliferation and terminal differentiation. Mechanistically, mitochondrial oxidative phosphorylation complexes were severely impaired in the Mto1 cKO fetal liver, and this was followed by cytosolic iron accumulation. Overloaded cytosolic iron promoted heme biosynthesis, which induced an unfolded protein response (UPR) in Mto1 cKO erythroblasts. An iron chelator or UPR inhibitor rescued erythroid terminal differentiation in the Mto1 cKO fetal liver in vitro. This mitochondrial regulation of iron homeostasis revealed the indispensable role of mitochondrial tRNA modification in fetal hematopoiesis.
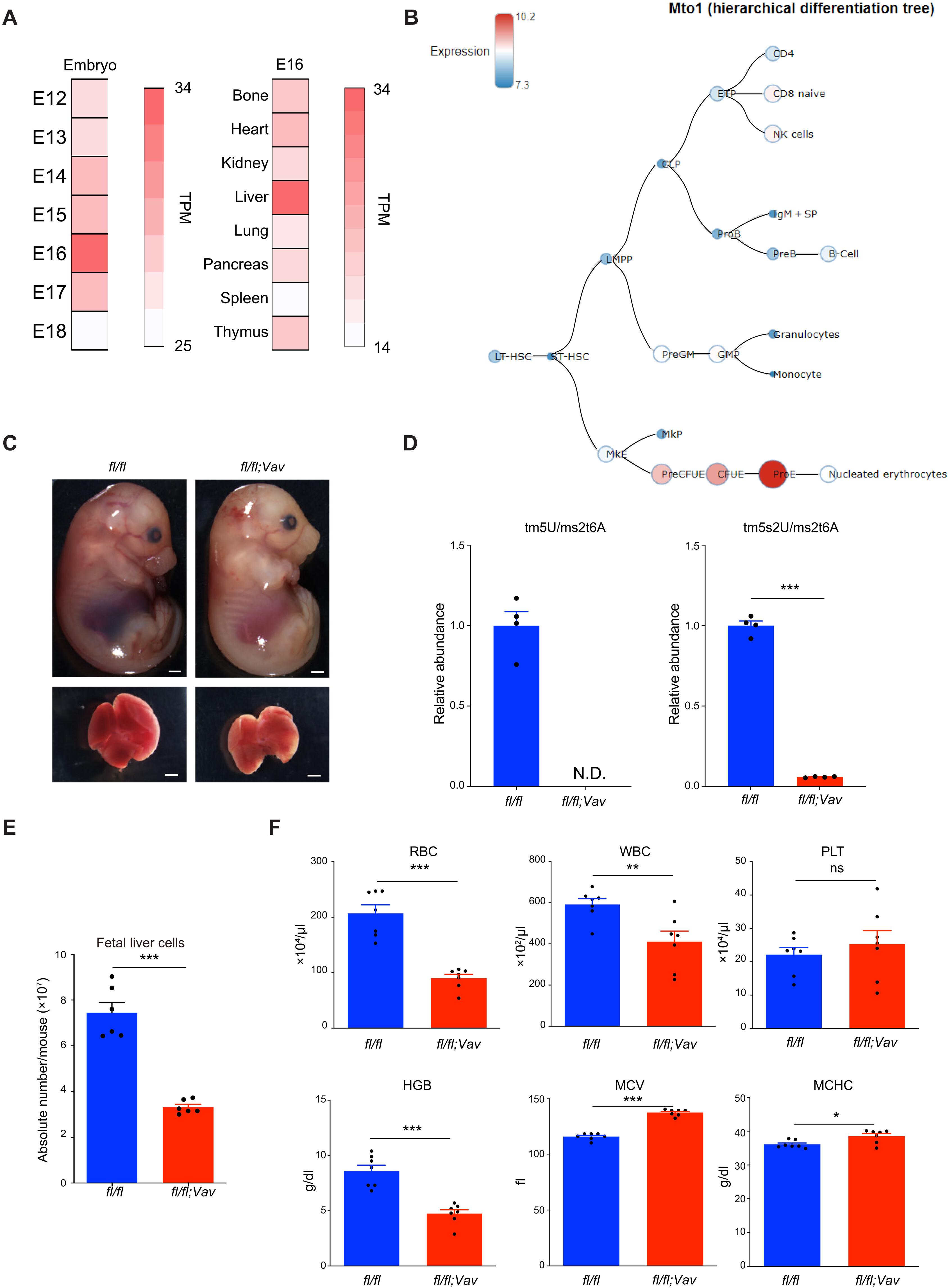
线粒体翻译通过维持铁平衡调节红细胞的末期分化
由线粒体tRNA翻译优化1 (Mto1)介导的线粒体tRNA牛磺酸修饰对线粒体蛋白翻译至关重要。Mto1缺乏可诱导胚胎干细胞的蛋白应激。最近的一项研究发现,一名MTO1基因突变的患者出现了严重的贫血,这使我们假设MTO1功能障碍可能导致红细胞生成缺陷。造血特异性Mto1条件敲除(cKO)小鼠是胚胎致死性的,并且在红母细胞增殖和终末分化方面表现出不依赖于生态位的缺陷。在机制上,线粒体氧化磷酸化复合物在Mto1 cKO胎儿肝脏中严重受损,随后是细胞质铁积累。过量的胞质铁促进血红素生物合成,从而诱导Mto1 cKO红母细胞的未折叠蛋白反应(UPR)。铁螯合剂或UPR抑制剂在体外挽救了Mto1 cKO胎儿肝脏的红系末端分化。线粒体对铁稳态的调节揭示了线粒体tRNA修饰在胎儿造血中不可或缺的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


