Self-Assembly of H2S-Generating Photosensitizer for Gas-Assisted Synergistic Photothermal Therapy
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
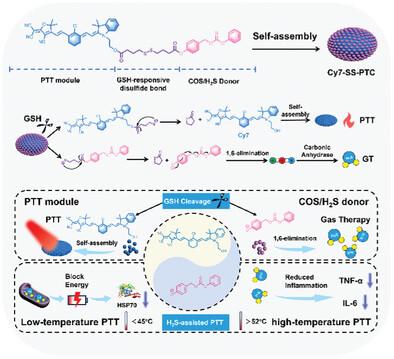
Photothermal therapy (PTT) is emerging as a promising cancer treatment, but uneven heat distribution increases side effects and reduces treatment precision, where high-temperature zones risk inducing undesired inflammation, while low-temperature regions are insufficient due to upregulation of heat shock proteins (HSPs). Herein, a gas-assisted PTT strategy is designed to link near-infrared heptamethine cyanine (Cy7) with self-immolative phenyl thiocarbonate (PTC), a hydrogen sulfide (H2S) donor through a disulfide bond, creating a small-molecule photosensitizer (Cy7-SS-PTC) that can self-assemble into nanoparticles (NPs) without stabilizers. Upon internalized by cancer cells, Cy7-SS-PTC NPs respond to elevated glutathione levels, and simultaneously release Cy7 and H2S via a cascade reaction. The released Cy7 reassembles into nanoaggregates, generating hyperthermia under 808 nm light irradiation, and then binds to albumin, producing strong near-infrared fluorescence to track tumors for precise treatment. The released H2S not only disrupts the mitochondrial respiratory chain, blocks ATP production, and suppresses HSP70 overexpression to amplify the efficacy of low-temperature PTT regions but also curbs proinflammatory cytokines in high-temperature PTT zones, delivering powerful tumor ablation with minimal inflammation. This small-molecule-based “H2S-assisted PTT” strategy optimizes the current PTT and validates its potential clinical application.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


