Promoting Efficient Ruthenium Sites With Lewis Acid Oxide for the Accelerated Hydrogen and Chlor-Alkali Co-Production
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
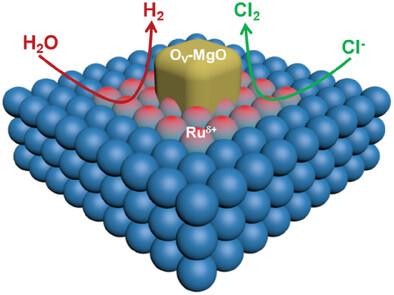
Ruthenium (Ru) –based catalysts have been considered a promising candidate for efficient sustainable hydrogen and chlor-alkali co-production. Theoretical calculations have disclosed that the hollow sites on the Ru surface have strong adsorption energies of H and Cl species, which inevitably leads to poor activity for cathodic hydrogen evolution reaction (HER) and anodic chlorine evolution reaction (CER), respectively. Furthermore, it have confirmed that anchoring Lewis acid oxide nanoparticles such as MgO on the Ru surface can induce the formation of the onion-like charge distribution of Ru atoms around MgO nanoparticles, thereby exposing the Ru-bridge sites at the interface as excellent H and Cl adsorption sites to accelerate both HER and CER. Under the guidance of theoretical calculations, a novel dispersed MgO nanoparticles on Ru (MgOx-Ru) electrocatalyst is successfully prepared. In strongly alkaline and saline media, MgOx-Ru recorded excellent HER and CER electrocatalytic activity with a very low overpotential of 19 mV and 74 mV at the current density of 10 mA cm−2, respectively. More stirringly, the electrochemical test with MgOx-Ru as both anodic and cathodic electrodes under simulated chlor-alkali electrolysis conditions demonstrated superior electrocatalytic performance to the industrial catalysts of commercial 20 wt% Pt/C and dimensionally stable anode (DSA).
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


