Bilateral Synergistic Effects of Phototherapy-Based NIR-II Absorption Photosensitizer for Allergic Rhinitis
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
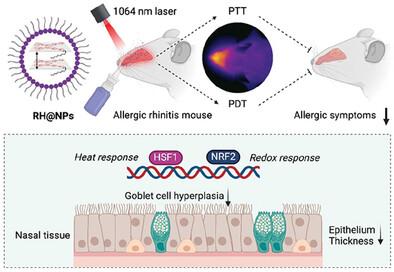
Allergic rhinitis (AR) is the most prevalent global health issue, affecting approximately 3 billion people, with its incidence increasing annually. The current first-line pharmacotherapy for symptom relief has limited efficacy and often results in notable side effects. Here, aza-BODIPY-based nanoparticles (RH@NPs) are developed that exhibit mild photothermal therapy (PTT) and type I photodynamic therapy (PDT) capabilities. Enhanced intramolecular charge transfer induces NIR-II absorption of the photosensitizer (RH), facilitating deeper tissue penetration for augmented AR therapy. Additionally, the use of an asymmetric donor–acceptor–acceptor′ configuration promotes the self-assembly of RH, enhancing its intersystem crossing ability and enabling efficient photophysical activity. The synergistic effects of PTT (enhancing HSF1 DNA-binding activity to inhibit epithelial-mesenchymal transition by epigenetically regulating the expression of epithelial-mesenchymal transition-associated genes) and PDT (activating NRF2 transcriptional activity to stimulate the antioxidant defense system) enable RH@NPs to provide a superior therapeutic effect in a mouse model of AR. This effect is achieved by mechanically reducing the allergic response rather than merely alleviating symptoms. Notably, the photosensitizer-based physical therapy demonstrates enhanced safety. This study is the first to successfully investigate the application of phototherapy for AR and elucidate its mechanism of action, offering a novel, straightforward, and efficient treatment strategy for AR.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


