Synergetic dual co-catalyst and interfacial bonds in N-CQDs/NCN/BNQDs to promote photoremoval of uranium(VI)
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
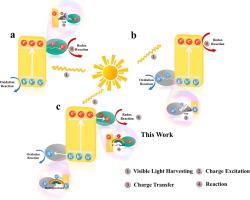
The photocatalytic efficiency of a catalyst is dependent on two main factors: the separation of excitons and the migration of photogenerated carriers. Therefore, we employed a strategy of defect engineering, co-catalyst co-modification and interfacial chemical bonding synergistic modification to prepare N-CQDs-x/NCN/BNQDs-y by introducing nitrogen defects in graphitic carbon nitride (NCN) and concomitantly incorporating boron nitride quantum dots (BNQDs) and nitrogen-doped carbon quantum dots (N-CQDs) onto its surface. And the N-CQDs-15/NCN/BNQDs-1 showed excellent photocatalytic removal of U(VI), which achieved 98.7 % after 120 min of illumination. Furthermore, the catalytic activities were ranked as follows: N-CQDs-15/NCN/BNQDs-1 > N-CQDs-15/NCN > NCN/BNQDs-1 > NCN > g-C3N4. Systematic investigations unveiled that the improved catalytic performance of N-CQDs-15/NCN/BNQDs-1 was mainly attributed to the nitrogen defects in g-C3N4 facilitating carrier separation, the C–C interface bonding between N-CQDs and g-C3N4 and the hydrogen bonding between BNQDs and g-C3N4, as well as co-catalysts transferring electrons and holes, respectively, promoting the simultaneous transfer of e- and h+. Simultaneously, the prepared photocatalytic materials exhibited outstanding cyclic stability, with the U(VI) removal rate by the catalyst remaining above 91 % after five consecutive photocatalytic experiments. This study not only presents an effective photocatalytic material for the remediation of uranium-containing wastewater but also offers insights into the design of efficient photocatalysts.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


