Electron Density Engineering at the Bond Critical Points in Solvation Sheath of Sodium Ions for High-Rate Hard Carbon in Ether-Based Electrolyte
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
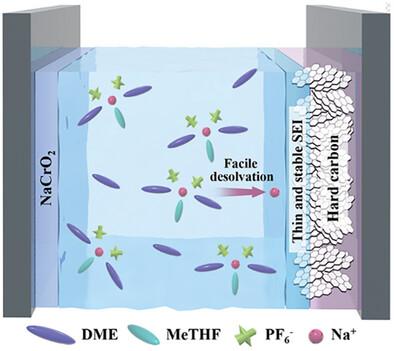
Rationally designing the electrolyte system toward improving the electrochemical performance, especially the rate capability, of sodium ion batteries (SIBs) is very important for accelerating their large-scale commercialization. Herein, it is shown that by refining the molar ratio of two ether solvents, namely dimethoxyethane (DME) and 2-methyl tetrahydrofuran (MeTHF), a binary solvent electrolyte system forms a sodium ion solvation structure that facilitates high rate charge/discharge of hard carbon (HC) electrodes. It is demonstrated that the boosted rate capability can be attributed to the enhanced sodium ion transportation and desolvation kinetics, resulting from the participation of weak-coordinating MeTHF molecule with low steric hindrance in the sodium ion solvation sheath, which weakens the interaction between sodium ion and solvent molecules/anions through electron density regulation at the bond critical points (BCPs). The thin and uniform solid electrolyte interphase film on HC electrodes formed in such an ether-based electrolyte is also beneficial for improving the rate performance and cycling stability. The results of the present study shed more light on how the electron density engineering at the BCPs in sodium ion solvation sheath affects the rate capability of HC electrodes and promote its practical application prospect in future sodium-based battery chemistries.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


