Environmental fate and ecotoxicity of diclofenac degradation products generated by photo-assisted advanced oxidation processes
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
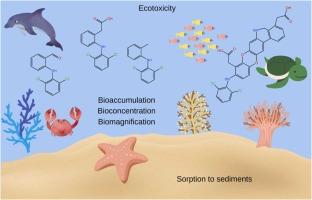
Diclofenac (DCF), a widely used non-steroidal anti-inflammatory drug (NSAID), poses environmental concerns due to its persistence, bioaccumulation potential, and transformation into toxic byproducts during oxidative and chlorination processes. This study investigated the photodegradation of DCF, both directly and in the presence of oxidants, to characterize the resulting degradation products and assess their potential environmental impact.The highest efficiency for direct UV photodegradation of DCF was observed at pH 5, while the addition of oxidants significantly accelerated the degradation rate. Among the advanced oxidation processes (AOPs) examined, the H₂O₂/UV system, with a DCF:H₂O₂ molar ratio of 1:30, exhibited the most effective performance in terms of DCF removal and total organic carbon (TOC) reduction. However, ecotoxicity assessments using Alivibrio fischeri, Daphnia magna, and Lemna minor revealed that AOPs generally increased the toxicity of the resulting solutions compared to untreated DCF.Toxicity analyses showed that post-reaction mixtures from AOPs involving NaOCl exhibited the highest toxic effects, consistent with forming specific transformation products identified as highly toxic by ECOSAR modeling. Additionally, the analysis of the physicochemical properties of DCF and its transformation products, including solubility and organic matter affinity, suggests a limited potential for long-range transport. These compounds are more likely to bind to sediments, reducing their mobility in groundwater.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


