A nucleosome switch primes hepatitis B virus infection
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
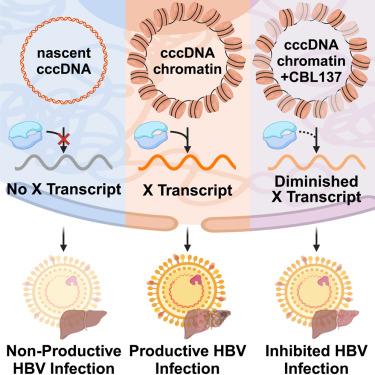
Chronic hepatitis B virus (HBV) infection is an incurable pathogen responsible for causing liver disease and hepatocellular carcinoma. During the genesis of infection, HBV establishes an independent minichromosome consisting of the viral covalently closed circular DNA (cccDNA) genome and host histones. The viral X gene must be expressed immediately upon infection to induce degradation of the host silencing factor, the Smc5/6 complex. However, the relationship between cccDNA chromatinization and X gene transcription remains poorly understood. By establishing a reconstituted viral minichromosome platform, we found that nucleosome occupancy in cccDNA regulates X transcription. We corroborated these findings in situ and further showed that the chromatin-destabilizing molecule CBL137 inhibits full-length X transcription and HBV infection in primary human hepatocytes. Our results shed light on a long-standing paradox and represent a potential therapeutic approach for the treatment of chronic HBV infection.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


