Systems immunology analysis of human immune organoids identifies host-specific correlates of protection to different influenza vaccines
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
Vaccines are an essential tool to significantly reduce pathogen-related morbidity and mortality. However, our ability to rationally design vaccines and identify correlates of protection remains limited. Here, we employed an immune organoid approach to capture human adaptive immune response diversity to influenza vaccines and systematically identify host and antigen features linked to vaccine response variability. Our investigation identified established and unique immune signatures correlated with neutralizing antibody responses across seven different influenza vaccines and antigens. Unexpectedly, heightened ex vivo tissue frequencies of T helper (Th)1 cells emerged as both a predictor and a correlate of neutralizing antibody responses to inactivated influenza vaccines (IIVs). Secondary analysis of human public data confirmed that elevated Th1 signatures are associated with antibody responses following in vivo vaccination. These findings demonstrate the utility of human in vitro models for identifying in vivo correlates of protection and establish a role for Th1 functions in influenza vaccination.
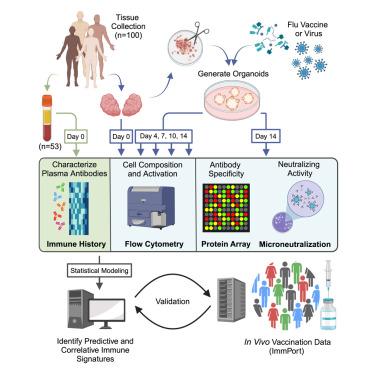
人类免疫类器官的系统免疫学分析确定了对不同流感疫苗保护的宿主特异性相关因子
疫苗是显著降低病原体相关发病率和死亡率的重要工具。然而,我们合理设计疫苗和确定保护相关因素的能力仍然有限。在这里,我们采用免疫类器官方法来捕获人类对流感疫苗的适应性免疫反应多样性,并系统地识别与疫苗反应变异性相关的宿主和抗原特征。我们的研究确定了与七种不同流感疫苗和抗原的中和抗体反应相关的已建立和独特的免疫特征。出乎意料的是,T辅助性(Th)1细胞的体外组织频率升高,既是对灭活流感疫苗(IIVs)中和抗体反应的预测因子,也是相关因子。对人类公开数据的二次分析证实,体内接种疫苗后,Th1信号升高与抗体应答有关。这些发现证明了人类体外模型在识别体内保护相关因素方面的实用性,并确立了Th1功能在流感疫苗接种中的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


