Mechano-oncogenic cytoskeletal remodeling drives leukemic transformation with mitochondrial vesicle-mediated STING activation
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
Mitochondria are integrated within the cytoskeleton for structural integrity and functional regulation, yet the pathological exploitation of these interactions in cell fate decisions remains largely unexplored. Here, we identify a cytoskeleton-mitochondria remodeling mechanism underlying leukemic transformation by the core-binding factor subunit beta and smooth muscle myosin heavy-chain fusion (CBFβ-SMMHC). This chimera reconstructs a cytosolic filamentous cytoskeleton, inducing NMIIA phosphorylation and INF2-dependent filamentous actin (F-actin) assembly, which enhance cellular stiffness and tension, leading to calcium-mediated mitochondrial constriction, termed cytoskeletal co-option of mitochondrial constriction (CCMC). CCMC can also be triggered through diverse approaches independent of CBFβ-SMMHC, reconstructing a similar cytoskeleton and recapitulating acute myeloid leukemia (AML) with consistent immunophenotypes and inflammatory signatures. Notably, CCMC generates TOM20−PDH+mtDNA+ mitochondrial-derived vesicles that activate cGAS-STING signaling, with Sting knockout abrogating CCMC-induced leukemogenesis. Targeted inhibition of CCMC or STING suppresses AML propagation while sparing normal hematopoiesis. These findings establish CCMC as an intrinsic mechano-oncogenic process linking genetic mutations with cytoskeletal remodeling to oncogenic transformation, highlighting its promise as a therapeutic target.
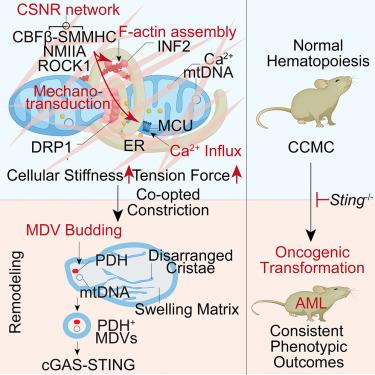
机械致癌细胞骨架重塑通过线粒体囊泡介导的STING激活驱动白血病转化
线粒体整合在细胞骨架内,以实现结构完整性和功能调节,但这些相互作用在细胞命运决定中的病理利用仍未得到充分探索。在这里,我们通过核心结合因子β亚基和平滑肌肌球蛋白重链融合(cbf - β- smmhc)确定了白血病转化背后的细胞骨架-线粒体重塑机制。该嵌合体重建了细胞质丝状细胞骨架,诱导NMIIA磷酸化和inf2依赖性丝状肌动蛋白(F-actin)组装,从而增强细胞刚度和张力,导致钙介导的线粒体收缩,称为细胞骨架线粒体收缩共选择(CCMC)。CCMC也可以通过独立于CBFβ-SMMHC的多种途径触发,重建类似的细胞骨架,重现具有一致免疫表型和炎症特征的急性髓性白血病(AML)。值得注意的是,CCMC产生TOM20−PDH+mtDNA+线粒体来源的囊泡,激活cGAS-STING信号,Sting敲除可消除CCMC诱导的白血病发生。靶向抑制CCMC或STING抑制AML的繁殖,同时保留正常的造血功能。这些发现表明,CCMC是一个内在的机械致癌过程,将细胞骨架重塑的基因突变与致癌转化联系起来,突出了其作为治疗靶点的前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


