Leptin enhances the intracellular thyroid hormone activation in skeletal muscle to boost energy balance
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Thyroid hormones (THs) are key modulators of energy metabolism and cross-talk with other endocrine and metabolic factors. Notably, leptin can increase hypothalamic control of TH synthesis as an adaptive metabolic response regulating body weight. In this study, we found that the TH signal is heightened in overweight humans and is lost with obesity. In mice, systemic and intracerebroventricular leptin injection induces the expression of type 2 deiodinase (D2), the TH-activating enzyme, in skeletal muscle. Mechanistically, leptin enhances the transcription of D2 by a STAT3- and α-melanocyte-stimulating hormone (α-MSH)/cyclic AMP (cAMP)-dependent regulation. Notably, mice lacking D2 or with a mutation in the TH receptor do not exhibit the metabolic effects of leptin, such as increased insulin sensitivity and oxygen consumption, indicating that leptin’s peripheral metabolic effects in skeletal muscle are mediated by TH. These findings underscore the critical role of leptin in integrating the TH-induced metabolic activation, while also contributing to appetite suppression in response to perceived fat stores.
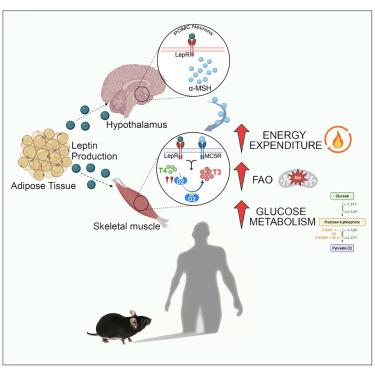
瘦素增强骨骼肌细胞内甲状腺激素的激活,促进能量平衡
甲状腺激素(THs)是能量代谢的关键调节剂,并与其他内分泌和代谢因子相互作用。值得注意的是,瘦素可以增加下丘脑对TH合成的控制,作为调节体重的适应性代谢反应。在这项研究中,我们发现TH信号在超重人群中升高,而随着肥胖而消失。在小鼠中,全身和脑室内注射瘦素可诱导骨骼肌中th激活酶2型去碘酶(D2)的表达。从机制上讲,瘦素通过STAT3-和α-促黑素细胞激素(α-MSH)/环AMP (cAMP)依赖性调节来增强D2的转录。值得注意的是,缺乏D2或TH受体突变的小鼠没有表现出瘦素的代谢作用,例如增加胰岛素敏感性和耗氧量,这表明瘦素在骨骼肌中的外周代谢作用是由TH介导的。这些发现强调了瘦素在整合th诱导的代谢激活中的关键作用,同时也有助于对感知到的脂肪储存的食欲抑制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


