Caloric restriction prevents inheritance of polycystic ovary syndrome through oocyte-mediated DNA methylation reprogramming
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Polycystic ovary syndrome (PCOS) is a prevalent metabolic and reproductive endocrine disorder with strong heritability. However, the independent role of oocytes in mediating this heritability remains unclear. Utilizing in vitro fertilization-embryo transfer and surrogacy, we demonstrated that oocytes from androgen-exposed mice (F1) transmitted PCOS-like traits to F2 and F3 generations. Notably, caloric restriction (CR) in F1 or F2 effectively prevented this transmission by restoring disrupted DNA methylation in oocyte genes related to insulin secretion and AMPK signaling pathways. Further detection in adult tissues of offspring revealed dysregulated DNA methylation and expression of those genes (e.g., Adcy3, Gnas, and Srebf1) were reversed by maternal CR. Moreover, similar benefits of CR were observed in aberrant embryonic methylome of women with PCOS. These findings elucidate the essential role of CR in preventing PCOS transmission via methylation reprogramming, emphasizing the importance of preconception metabolic management for women with PCOS.
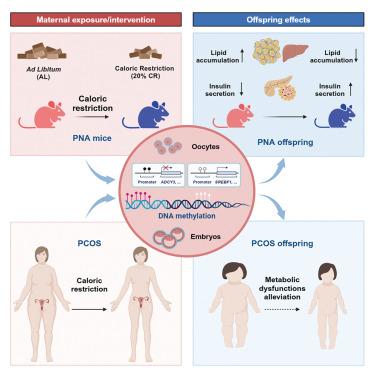
热量限制通过卵母细胞介导的DNA甲基化重编程阻止多囊卵巢综合征的遗传
多囊卵巢综合征(PCOS)是一种常见的代谢和生殖内分泌疾病,具有很强的遗传性。然而,卵母细胞在介导这种遗传性中的独立作用仍不清楚。利用体外受精-胚胎移植和代孕,我们证明雄激素暴露小鼠(F1)的卵母细胞将pcos样性状遗传给F2和F3代。值得注意的是,F1或F2的热量限制(CR)通过恢复与胰岛素分泌和AMPK信号通路相关的卵母细胞基因中被破坏的DNA甲基化,有效地阻止了这种传递。在后代成年组织中进一步检测发现,DNA甲基化失调,这些基因(如Adcy3、Gnas和Srebf1)的表达被母体CR逆转。此外,CR在PCOS女性胚胎甲基化异常中也有类似的益处。这些发现阐明了CR在通过甲基化重编程预防PCOS传播中的重要作用,强调了孕前代谢管理对PCOS女性的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


