Structural basis for the RNA binding properties of mouse IGF2BP3
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
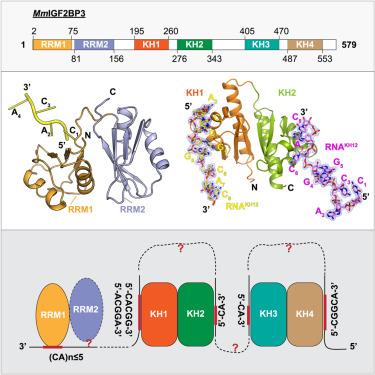
IGF2BP family proteins (IGF2BPs) contain six tandem RNA-binding domains (RBDs), resulting in highly complex RNA binding properties. Dissecting how IGF2BPs recognize their RNA targets is essential for understanding their regulatory roles in gene expression. Here, we have determined the crystal structures of mouse IGF2BP3 constructs complexed with different RNA substrates. Our structures reveal that the IGF2BP3-RRM12 domains can recognize CA-rich elements up to 5-nt in length, mainly through RRM1. We also captured the antiparallel RNA-binding mode of the IGF2BP3-KH12 domains, with five nucleotides bound by KH1 and two nucleotides bound by KH2. Furthermore, our structural and biochemical studies suggest that the IGF2BP3-KH12 domains could recognize the “zipcode” RNA element within the β-actin mRNA. Finally, we analyzed the similarities and differences of the RNA-binding properties between the KH12 and KH34. Our studies provide structural insights into RNA target recognition by mouse IGF2BP3.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


