High-Throughput Screening Identifies Anionic Polymer Supports that Improve Enzyme Activity at Low pH and High Temperature
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
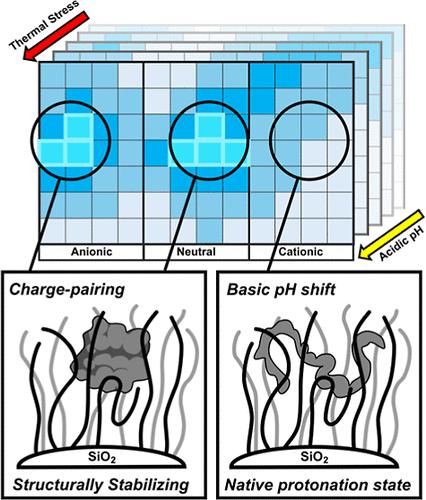
Elevated temperatures and nonoptimal pH can destabilize enzyme structure or change the protonation state of catalytic residues resulting in attenuated catalytic performance. Enzyme immobilization on polymer supports enables the fine-tuning of highly varied vicinal chemistries to improve enzyme performance by promoting correctly folded enzyme structure and adjusting the local microenvironment to more favorable conditions. Herein, we sought to investigate how multicomponent random copolymer brushes composed of monomers with anionic, cationic, neutral (zwitterionic, and mixed-charge), and aromatic properties stabilize covalently tethered lipase A fromBacillus subtilis at low pH and high temperature. Polymer brush compositions were screened using a high-throughput approach involving the combinatorial synthesis of random copolymer brushes and in situ characterization of immobilized lipase function. Although cationic supports provided a modest improvement over soluble lipase in maximum activity and thermal stability at low pH, more substantial enhancements in lipase stability were observed for anionic and neutral zwitterionic polymer supports, resulting in increases in temperature optima as great as 40 °C (from 40 to 80 °C) and an increase in maximum activity by more than 300%. These observations were counter to expectations regarding the role of surface charge on local pH and were attributed instead to the preservation of enzyme structure due to stabilizing electrostatic interactions between negatively charged polymer moieties and the net positively charged surface of lipase A. Our findings suggest that the stabilization of enzyme structure by charged polymers may offset unfavorable local changes in pH in certain situations, while in other situations these effects may be synergistic.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


