Synthesis and Characterization of MO:ZnO/Fe2O3 Nanocomposite and Its Effectiveness in the Degradation of Green Malachite Dye: Molecular Dynamics and Electronic Properties Study of Green Malachite Adsorption
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
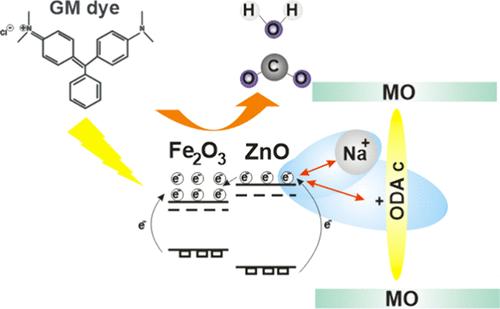
Nanocomposites have attracted significant attention from researchers due to their remarkable chemical, adsorptive, and thermal properties. This work focuses on the synthesis of the montmorillonite modified with octadecylamine (MO):ZnO/Fe2O3 (MO:ZnO/Fe2O3) nanocomposite. The ZnO/Fe2O3 nanocomposite and MO were mixed in solution to create the component. XRD, FTIR, BET, TEM, MEB, and UV–vis were used to characterize the materials. In terms of their textural, morphological, and structural characteristics, interesting results were found. After 30 min of sunlight exposure, 96.12% of the GM dye can be degraded using just 0,02 g of MO:ZnO/Fe2O3 (1:2(1/0.05)). In contrast, ZnO NPs exhibited the highest percentage of degradation under UV light, achieving 91.85%. The greater efficiency of MO:ZnO/Fe2O3 under sunlight is attributed to its narrower band gap of 2.25 eV, which enables better utilization of visible light. Next using the Forcite and CASTEP modules in the Material Studio software, the GM dye’s adsorption behavior on the surface of the as-prepared nanocomposite was analyzed. The results demonstrate that the GM/MO interaction is of the chemisorption type, predominantly governed by hydrogen bonding, electrostatic interactions, and π–π interactions between GM molecules. The ZnO (100) surface exhibits the highest density of active sites for GM degradation via a chemisorption adsorption process. Molecular dynamics simulations at 289.15 K reveal that the redox processes responsible for the degradation of the GM pollutant and its conversion into CO2 gas are exothermic. For ZnO, the electronic properties yield a band gap of 2.686 eV. For GM/ZnO (100) and GM/ZnO (101), the band gaps were determined to be 0.291 and 2.704 eV, respectively, by using a 340 eV cutoff energy and the GGA RPBE pseudofunctional.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


