Lewis Acid-Enabled Chemodivergent Cycloadditions of Donor–Acceptor Cyclopropanes with Indoline-2-Thiones
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
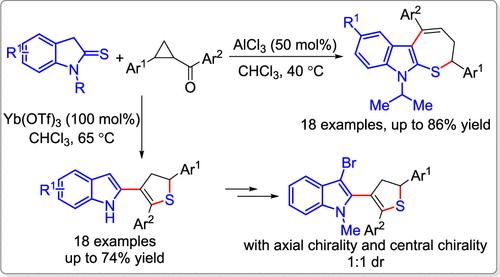
Lewis acid-enabled reactions of donor–acceptor cyclopropanes (DACs) with indoline-2-thiones are reported. The reaction exhibits tunable annulation depending on the Lewis acid and the substituent at N1 of the indoline-2-thiones. With AlCl3 as the Lewis acid and 1-isopropylindoline-2-thiones as reactants, a direct ring opening with DACs, followed by intramolecular nucleophilic addition/dehydration takes place leading to the formation of dihydro-2H-thiepino[2,3-b]indoles in moderate to good yields. Using Yb(OTf)3 as promoter and 1-unsubstituted indoline-2-thiones as reactants, a (3 + 2) cycloaddition with DACs accompanied by sulfur rearrangement nucleophilic addition/dehydration takes place to give 3-indolyl-4,5-dihydrothiophenes in moderate yields. In addition, the synthetic transformation of 3-indolyl-4,5-dihydrothiophene to sulfone and indole-based axially chiral scaffolds further extends the synthetic utility and structural complexity.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


