Chemoproteomic Profiling of a Carbon-Stabilized Gold(III) Macrocycle Reveals Cellular Engagement with HMOX2
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
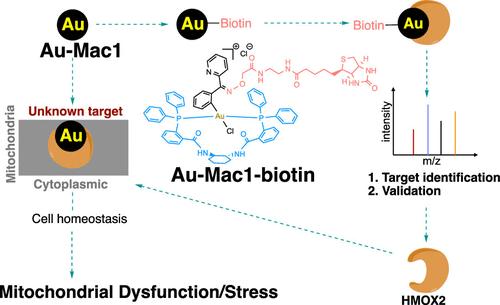
In this work, we discovered a novel organometallic gold(III) macrocycle, Au-Mac1, that demonstrates anticancer potency in a panel of triple-negative breast cancer cells (TNBC), and based on this complex, a biotinylated-Au-Mac1 probe was designed for target identification via chemoproteomics, which uncovered the engagement of HMOX2 of the heme-energy metabolism pathway. Using orthogonal chemical biology and molecular biology approaches, including immunoblotting, flow cytometry, and cellular thermal shift assays, it was confirmed that Au-Mac1 engages HMOX2 in cells. Downstream effects of Au-Mac1 on the depletion of mitochondrial membrane proteins and bioenergetics point to the potential role of HMOX2 in cancer. Importantly, Au-Mac1 inhibits in vivo tumor growth of metastatic breast tumor-bearing mice. We believe that this approach is clinically relevant in network-oriented drug discovery. To the best of our knowledge, Au-Mac1 is the first gold complex that targets HMOX2 to elicit an anticancer effect.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


