Flexible Intervention of Polyoxometalate Support on the Electronegativity of Single Atoms to Enhance Catalytic Activity
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
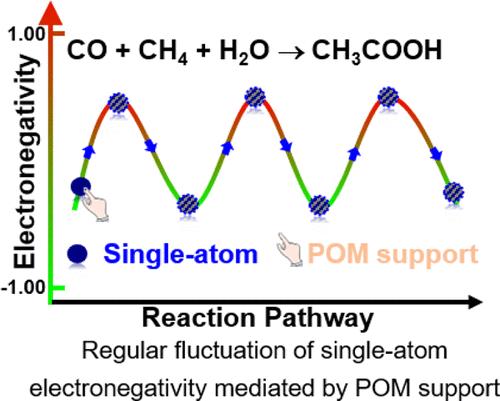
The construction of single-atom catalysts (SACs) using polyoxometalates (POMs) as supports has attracted significant attention. Specifically, POMs possess the unique ability to reversibly accept and donate electrons; yet, the potential benefits of this distinctive characteristic on the activity of single atoms have remained unexplored. In this study, we employ density functional theory (DFT) calculations to investigate the synthesis of CH3COOH from CO, CH4, and H2O catalyzed by POM-supported SAC M1/POM (M = Pt, Rh, Ru, Pd, Co), aiming to gain a more comprehensive understanding of the role of POM support in SACs. Our proposed mechanism first involves CH4 activation for producing •CH3 and, at the same time, the catalytic intermediate [M1/POM]−. Then, CO and •CH3 are sequentially adsorbed on the single-atom site of [M1/POM]− and coupled to form COCH3. Finally, as H2O attacks CH3CO, CH3COOH is formed and released. The poorer activity of M1/POM (M = Rh, Ru, Pd, Co) compared with that of Pt1/POM is attributed to the low matching degree in the frontier molecular orbital energy between [M1/POM]− and CO/•CH3, which results in the inaccessibility of CO and •CH3 adsorptions, thus hindering the subsequent CH3COOH formation. Throughout the reaction process, the POM support promotes dynamic switching of single atoms between electron-rich and electron-poor states, leveraging its reversible electron transfer capability. The electron-deficient species •CH3 adsorption and H2O attack are enabled by the regular access of single atoms to electron-saturated state, while timely switching of single atoms to electron-deficient states facilitates CO adsorption and CH3 attack.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


