Sulfur Defect Tuning of CuS/BiVO4 and Understanding the Function of Hydrogen Radicals for Photoelectrochemical Ammonia Production
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
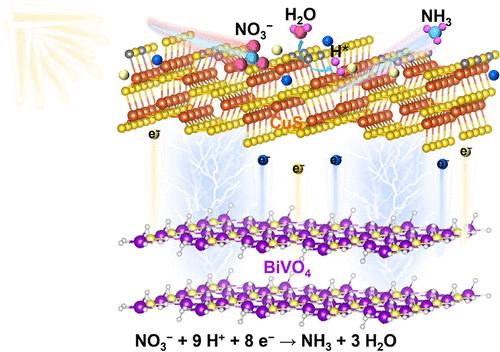
Photoelectrochemical nitrate reduction has been a promising method for ammonia (NH3) production under normal temperatures and neutral conditions. However, hydrogenation is a key process in the selective production of NH3 during nitrate reduction; therefore, inducing the active hydrogen for nitrate hydrogenation and inhibiting hydrogen production are a noteworthy problem. In this study, BiVO4/CuS (BVO/CS) heterostructure has been constructed for a photoelectrochemical nitrate reduction reaction (PEC NIRR). The introduction of CuS optimizes the electron-transfer ability and enhances the surface catalytic kinetics of BVO/CS. At the same time, the presence of sulfur vacancies on the surface promotes the adsorption and activation of nitrate, realizes the splitting of H2O, and successfully generates abundant hydrogen radicals (H*). The generated H* is effectively utilized in the hydrogenation of NIRR. The NH3 yield and selectivity of optimal BVO/CS reach 30.55 μg h–1 cm–2 and 43.8%, respectively, which are 2.65 and 2.39 times that of bare BVO. Therefore, this work determines the key role of H* for nitrate hydrogenation, providing a novel strategy for boosting PEC NIRR. CuS/BiVO4 was successfully fabricated for photoelectrochemical nitrate reduction. Sulfur defects enabled the generation of hydrogen radicals, which effectively promoted nitrate hydrogenation and NH3 production.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


