Monolithic Low-tortuous Copper(I) Phosphide Nanorod Arrays for Exceptional Areal Performance in Electrochemical Chloride Ion Removal
IF 16.8
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
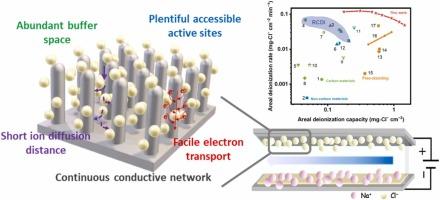
Copper(I) phosphide (Cu3P) with high theoretical capacity and metalloid characteristics performs great potential as chloride ion (Cl−) storage electrodes in chloride-ion batteries and deionization. However, the unsatisfactory electrochemical performance of Cu3P due to the sluggish rate and poor stability severely limited the development of practical application in electrochemical Cl− removal. Herein, monolithic low-tortuous Cu3P nanorod arrays (mCu3P NA) have been fabricated through sacrificial template array engineering combined with the controlled phosphating strategy. Benefiting from the low-tortuous array structural advantages involving rapid charge transfer and plentiful active sites, the resultant mCu3P NA electrode exhibited excellent areal deionization capacity (2.21 mg-Cl− cm−2 at 1.6 V) and corresponding fabulous rate (0.074 mg-Cl− cm−2 min−1), which was superior to previously reported electrodes in electrochemical deionization. Furthermore, the phosphating degree could adjust array architecture and component of Cu3P/Cu heterostructure, thus affecting areal deionization performance. In addition, the mechanism of Cl− capture by Cu3P was first elucidated, related to the redox of P3−/P0 and the formation of CuCl. These findings unlock the potential and elucidate the mechanism of Cu3P for electrochemical Cl− removal, as well as provide a new idea for rational design of high-performance array electrodes for environmental applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nano Energy
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
30.30
自引率
7.40%
发文量
1207
审稿时长
23 days
期刊介绍:
Nano Energy is a multidisciplinary, rapid-publication forum of original peer-reviewed contributions on the science and engineering of nanomaterials and nanodevices used in all forms of energy harvesting, conversion, storage, utilization and policy. Through its mixture of articles, reviews, communications, research news, and information on key developments, Nano Energy provides a comprehensive coverage of this exciting and dynamic field which joins nanoscience and nanotechnology with energy science. The journal is relevant to all those who are interested in nanomaterials solutions to the energy problem.
Nano Energy publishes original experimental and theoretical research on all aspects of energy-related research which utilizes nanomaterials and nanotechnology. Manuscripts of four types are considered: review articles which inform readers of the latest research and advances in energy science; rapid communications which feature exciting research breakthroughs in the field; full-length articles which report comprehensive research developments; and news and opinions which comment on topical issues or express views on the developments in related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


