Exhaustive reduction of aromatic esters and carboxylic acids with a homogeneous cobalt catalyst
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
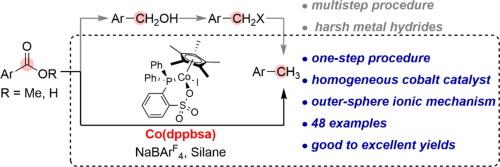
A one-step procedure to exhaustively reduce aromatic esters and carboxylic acids to their corresponding tolyl derivatives is a significant transformation in organic synthesis, which remains challenging in homogeneous catalysis. In this study, it’s achieved by using the well-defined phosphinesulfonate chelated cobalt-based catalyst (Co(dppbsa)), along with NaBArF4 as the basic additive and (MeO)2MeSiH as the reductant. Particularly, this cobalt catalysis proceeds through ether intermediates. Experimental combined theoretical results support it follows an outer-sphere ionic hydrosilylation mechanism and the in situ formed cationic siloxane species are involved in the rate-limiting hydride transfer. The same system also enables the catalytic deoxygenation of aliphatic esters, aliphatic acids, and amides but terminating at the corresponding ethers, alkanols and secondary amines, respectively. These findings open up novel avenues for the catalytic application of phosphinesulfonate chelated complexes, potentially serving as the foundation for the exhaustive reduction of carboxylic skeletons with cobalt.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


