Inflammasome mediated in situ cancer vaccine activated by schottky heterojunction for augmented immunotherapy
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
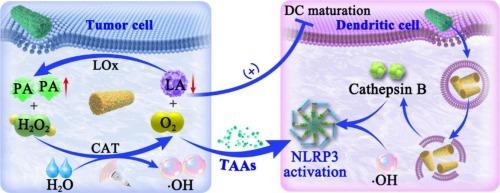
In situ cancer vaccines have emerged as an attractive paradigm for cancer immunotherapy. Nevertheless, insufficient antigens production, weak antigen presentation and immunosuppressive tumor microenvironment impeded the effectiveness of tumor immunotherapy. Herein, we constructed the NLRP3 inflammasome mediated in situ cancer vaccine (FPLB), in which rod shaped α-Fe2O3@Pt schottky heterojunction loaded with lactate oxidase (LOx) and surface-modified with bovine serum albumin and folic acid conjugation (FA-BSA). On the one hand, FPLB NPs utilizes its physicochemical properties of high aspect ratio to induce the breakdown of dendritic cells (DCs) lysosomes and the release of cathepsin B, thereby activating the NLRP3 inflammasome. Besides, the formation of “circulating pump” by harnessing catalase (CAT) activity and LOx activity could continuously consume lactic acid to alleviate the inactivation of cytokines induced by lactic acid excess, thereby transforming inflammatory activators into controllable nanoadjuvants. On the other hand, the “circulating pump” not only catalyze continuous generation of pyruvic acid to block the cell cycle, but also boosts charge utilization efficiency for excellent sonodynamic therapy (SDT) effect under ultrasound irradiation, thereby inducing the apoptosis or necrosis of tumor cells and releasing tumor-associated antigens (TAAs). FPLB demonstrates a significant NLRP3-mediated anti-tumor immune response both in vitro and in vivo. This strategy provides a new paradigm for the construction of NLRP3 inflammasome-mediated in situ cancer vaccines, which will have profound implications for the application of immunotherapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


