Using Common Alcohols to Transform N–H NHC-Palladium Complexes into Reactive Catalysts for the Suzuki–Miyaura Reaction with Aryl Chlorides
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2025-02-06
DOI:10.1021/acs.joc.4c0141110.1021/acs.joc.4c01411
引用次数: 0
Abstract
We demonstrate that the generation of mixed NHC/phosphinite Pd catalysts from 2-phosphinoimidazole ligands using bulky alcohols provides highly active Pd catalysts for cross-coupling reactions. Mechanistic studies suggest that the rapid loss of bulkier phosphinites from the precatalyst leads to more rapid oxidative addition. These catalysts demonstrate a broad substrate scope in Suzuki–Miyaura reactions with aryl chlorides. Conversion of a methanol-derived catalyst into a tert-butanol-derived catalyst in situ also enables selective and sequential cross-coupling of bromochloroarenes.
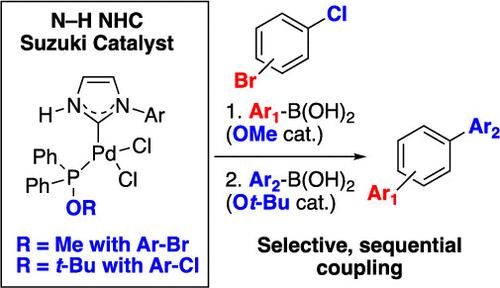
用普通醇将N-H nhc -钯络合物转化为芳基氯化物Suzuki-Miyaura反应催化剂
我们证明了用大体积醇从2-磷酰咪唑配体生成混合NHC/亚硝酸盐钯催化剂为交叉偶联反应提供了高活性的钯催化剂。机理研究表明,预催化剂中体积较大的亚磷酸盐的快速损失导致更快速的氧化加成。这些催化剂在与芳酰氯的Suzuki-Miyaura反应中显示出广泛的底物范围。甲醇衍生催化剂就地转化为叔丁醇衍生催化剂也使溴氯芳烃的选择性和顺序交叉偶联成为可能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


