RNA neoantigen vaccines prime long-lived CD8+ T cells in pancreatic cancer
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
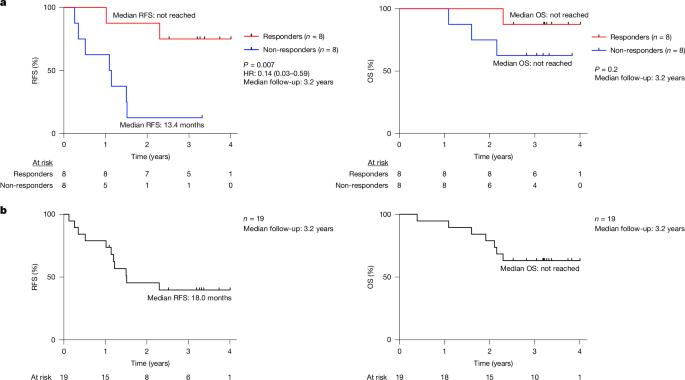
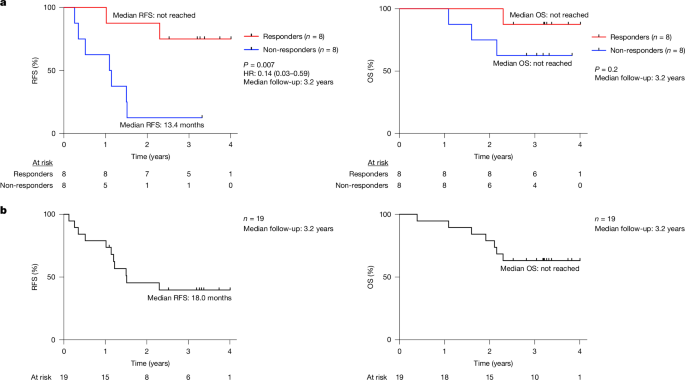
A fundamental challenge for cancer vaccines is to generate long-lived functional T cells that are specific for tumour antigens. Here we find that mRNA–lipoplex vaccines against somatic mutation-derived neoantigens may solve this challenge in pancreatic ductal adenocarcinoma (PDAC), a lethal cancer with few mutations. At an extended 3.2-year median follow-up from a phase 1 trial of surgery, atezolizumab (PD-L1 inhibitory antibody), autogene cevumeran1 (individualized neoantigen vaccine with backbone-optimized uridine mRNA–lipoplex nanoparticles) and modified (m) FOLFIRINOX (chemotherapy) in patients with PDAC, we find that responders with vaccine-induced T cells (n = 8) have prolonged recurrence-free survival (RFS; median not reached) compared with non-responders without vaccine-induced T cells (n = 8; median RFS 13.4 months; P = 0.007). In responders, autogene cevumeran induces CD8+ T cell clones with an average estimated lifespan of 7.7 years (range 1.5 to roughly 100 years), with approximately 20% of clones having latent multi-decade lifespans that may outlive hosts. Eighty-six percent of clones per patient persist at substantial frequencies approximately 3 years post-vaccination, including clones with high avidity to PDAC neoepitopes. Using PhenoTrack, a novel computational strategy to trace single T cell phenotypes, we uncover that vaccine-induced clones are undetectable in pre-vaccination tissues, and assume a cytotoxic, tissue-resident memory-like T cell state up to three years post-vaccination with preserved neoantigen-specific effector function. Two responders recurred and evidenced fewer vaccine-induced T cells. Furthermore, recurrent PDACs were pruned of vaccine-targeted cancer clones. Thus, in PDAC, autogene cevumeran induces de novo CD8+ T cells with multiyear longevity, substantial magnitude and durable effector functions that may delay PDAC recurrence. Adjuvant mRNA–lipoplex neoantigen vaccines may thus solve a pivotal obstacle for cancer vaccination. In a phase 1 trial, patients with pancreatic ductal adenocarcinoma who were treated with surgery and bespoke neoantigen mRNA vaccines combined with anti-PD-L1 and chemotherapy exhibited marked long-lived persistence of neoantigen-specific CD8+ T cell clones, which correlated with prolonged recurrence-free survival at a 3.2-year follow-up.
RNA新抗原疫苗在胰腺癌中激活长寿命的CD8+ T细胞
癌症疫苗的一个基本挑战是产生对肿瘤抗原具有特异性的长寿命功能性T细胞。在这里,我们发现针对体细胞突变衍生的新抗原的mrna -脂质体疫苗可能解决胰腺导管腺癌(PDAC)的这一挑战,PDAC是一种很少突变的致命癌症。在对PDAC患者进行手术、atezolizumab (PD-L1抑制抗体)、autogene cevumeran1(带有脊骨优化的尿嘧啶mrna -脂质体纳米颗粒的个体化新抗原疫苗)和改良的(m) FOLFIRINOX(化疗)的1期试验延长的中位随访中,我们发现疫苗诱导的T细胞应答者(n = 8)具有延长的无复发生存期(RFS;中位数未达到)与没有疫苗诱导T细胞的无应答者相比(n = 8;中位RFS为13.4个月;P = 0.007)。在应答者中,自基因cevumeran诱导CD8+ T细胞克隆,其平均估计寿命为7.7年(范围为1.5年至大约100年),其中约20%的克隆具有潜在的数十年寿命,可能比宿主寿命更长。每位患者86%的克隆在接种疫苗后约3年持续存在,包括对PDAC新表位具有高亲和力的克隆。使用一种新颖的计算策略来追踪单个T细胞表型,我们发现疫苗诱导的克隆在接种前组织中是检测不到的,并且假设在接种后长达三年的细胞毒性,组织驻留记忆样T细胞状态具有保留的新抗原特异性效应功能。两名应答者复发,证明疫苗诱导的T细胞较少。此外,将疫苗靶向癌症克隆的复发性pdac进行了修剪。因此,在PDAC中,自基因cevumeran诱导具有多年寿命、大量和持久效应功能的CD8+ T细胞新生,可能延迟PDAC复发。因此,佐剂mrna -脂质体新抗原疫苗可能解决癌症疫苗接种的关键障碍。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


