Mechanism for local attenuation of DNA replication at double-strand breaks
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
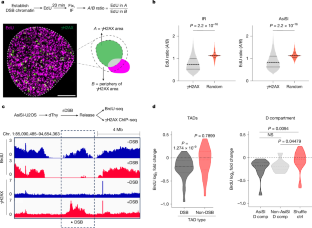
DNA double-strand breaks (DSBs) disrupt the continuity of the genome, with consequences for malignant transformation. Massive DNA damage can elicit a cellular checkpoint response that prevents cell proliferation1,2. However, how highly aggressive cancer cells, which can tolerate widespread DNA damage, respond to DSBs alongside continuous chromosome duplication is unknown. Here we show that DSBs induce a local genome maintenance mechanism that inhibits replication initiation in DSB-containing topologically associating domains (TADs) without affecting DNA synthesis at other genomic locations. This process is facilitated by mediators of replication and DSBs (MRDs). In normal and cancer cells, MRDs include the TIMELESS–TIPIN complex and the WEE1 kinase, which actively dislodges the TIMELESS–TIPIN complex from replication origins adjacent to DSBs and prevents initiation of DNA synthesis at DSB-containing TADs. Dysregulation of MRDs, or disruption of 3D chromatin architecture by dissolving TADs, results in inadvertent replication in damaged chromatin and increased DNA damage in cancer cells. We propose that the intact MRD cascade precedes DSB repair to prevent genomic instability, which is otherwise observed when replication is forced, or when genome architecture is challenged, in the presence of DSBs3–5. These observations reveal a previously unknown vulnerability in the DNA replication machinery that may be exploited to therapeutically target cancer cells. DNA double-strand breaks induce local genome maintenance and inhibition of replication initiation at nearby topologically associating domains without affecting global DNA synthesis.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


