MnO2-Assisted Photosynthetic Bacteria Interfering with the Adenosine-A2AR Metabolic Pathway to Enhance Tumor Photothermal Immunotherapy
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
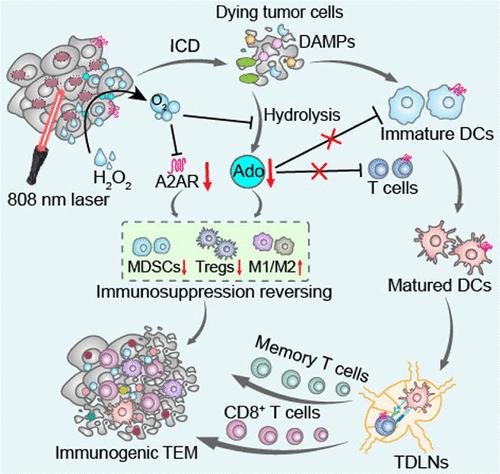
Hypoxia-related adenosine (Ado) exerts an immunosuppressive effect in tumors by binding to the metabolic checkpoint Ado A2A receptors (A2AR), thereby hindering the activation of antitumor immunity induced by immunogenic cell death (ICD). In this study, a MnO2-assisted photosynthetic bacteria (PSB) biohybrid (MnO2@PSB) is developed to enhance tumor photothermal immunotherapy by interfering with the Ado-A2AR metabolic pathway. Specifically, manganese dioxide (MnO2) nanoflowers are conjugated onto PSB by the carbodiimide reaction to construct the biohybrid MnO2@PSB. As a photothermal agent, MnO2@PSB generates heat to “burn” tumor cells under 808 nm laser irradiation, inducing tumor cell ICD. Meanwhile, MnO2@PSB catalyzes the decomposition of endogenous hydrogen peroxide into oxygen to alleviate tumor hypoxia, thereby reducing Ado production and downregulating the expression of A2AR, further reversing the tumor immunosuppressive microenvironment and amplifying the ICD effects. In various mouse 4T1 tumor models, MnO2@PSB can enhance antitumor immune responses, prolong mouse survival, and significantly inhibit tumor growth, recurrence, and metastasis under 808 nm laser irradiation. Collectively, this study provides a direction for enhanced antitumor immunotherapy through regulating metabolic pathways.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


