Synergistic role of gut-microbial L-ornithine in enhancing ustekinumab efficacy for Crohn’s disease
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
The role of the intestinal microbiome in Crohn’s disease (CD) treatment remains poorly understood. This study investigates microbe-host interactions in CD patients undergoing ustekinumab (UST) therapy. Fecal metagenome, metabolome, and host transcriptome data from 85 CD patients were analyzed using multi-omics integration and mediation analysis. Our findings reveal significant microbiome-metabolite-host interactions. Specifically, Faecalibacterium prausnitzii was linked to altered L-ornithine biosynthesis, resulting in higher L-ornithine levels in patients before UST therapy. In vivo and in vitro studies demonstrated that microbiome-derived L-ornithine enhances UST treatment sensitivity in CD by disrupting the host IL-23 receptor signaling and inhibiting Th17 cell stabilization through the IL-12RB1/TYK2/STAT3 axis. L-ornithine significantly enhances the therapeutic efficacy of UST in CD patients, as demonstrated in a prospective clinical trial. These findings suggest that targeting specific microbe-host metabolic pathways may improve the efficacy of inflammatory bowel disease (IBD) treatments.
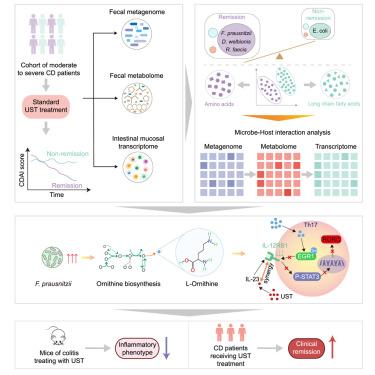
肠道微生物l -鸟氨酸在增强ustekinumab治疗克罗恩病疗效中的协同作用
人们对肠道微生物组在克罗恩病(CD)治疗中的作用仍然知之甚少。本研究调查了接受乌司替单抗(UST)治疗的克罗恩病患者体内微生物与宿主之间的相互作用。通过多组学整合和中介分析,对 85 名 CD 患者的粪便元基因组、代谢组和宿主转录组数据进行了分析。我们的研究结果揭示了微生物组-代谢组-宿主之间的重要相互作用。特别是,普氏粪杆菌(Faecalibacterium prausnitzii)与L-鸟氨酸生物合成的改变有关,导致患者在接受 UST 治疗前的 L-鸟氨酸水平较高。体内和体外研究表明,微生物衍生的L-鸟氨酸通过破坏宿主IL-23受体信号传导,并通过IL-12RB1/TYK2/STAT3轴抑制Th17细胞稳定,从而增强了CD患者对UST治疗的敏感性。在一项前瞻性临床试验中,L-鸟氨酸明显提高了UST对CD患者的疗效。这些研究结果表明,针对特定的微生物-宿主代谢途径可能会提高炎症性肠病(IBD)的疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


