A thiocoumarin based self-reporting sulfide prodrug strategy with a favorable safety profile
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
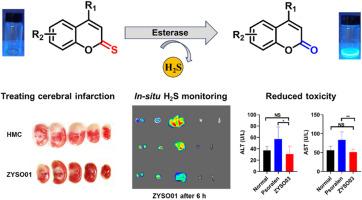
H2S as the third gasotransmitter is an important endogenous bioregulator that shows various therapeutic potentials. Herein, we present a novel thiocoumarin-based self-reporting sulfide prodrug strategy that utilizes esterase-mediated hydrolysis of thionoesters to release H2S and provide real-time fluorescence monitoring. Our key discovery is that thionoesters can be hydrolyzed by esterases to release H2S under physiological conditions, providing ample opportunities to design prodrugs based on ester-containing molecules. Thiocoumarin derivatives bearing a unique lactone structure offer advantages that simplify prodrug construction by substituting oxygen with sulfur in coumarin backbone and allow in-situ monitoring of H2S release through thiocoumarin-coumarin transformation. Our prodrug candidates are demonstrated with favorable H2S release kinetics and showed combined therapeutic effects of H2S and coumarin, making them promising for treating cerebral infarction. Fluorescent monitoring in mouse confirmed sustained H2S release and revealed the organ distribution, further validating the self-reporting system. Additionally, this approach that ensures therapeutic efficacy and reduces the hepatorenal toxicity of coumarin derivatives constitutes a facile prodrug strategy to overcome the toxicity of drug candidates.

求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


